10
Cell News 2/2015
“A sweet deal – modulation of amino sugar
metabolism affects protein quality and ageing”
Moritz Horn
1
, Kira Allmeroth
1,2
, Martin S. Denzel
1
1
Max Planck Institute for Biology of Ageing | Joseph-Stelzmann-Str. 9b | D-50931 Cologne | Germany
2
Cluster of Excellence in Cellular Stress Responses in Aging-associated Diseases (CECAD)
Joseph-Stelzmann-Str. 26 | D-50931 Cologne | Germany
contact:
When organisms age, the fidelity of molecular maintenance
mechanisms declines and cellular processes tightly controlled
in young age go astray. A prime example is protein homeosta-
sis, which includes the concerted interplay of protein synthesis,
-modification, -folding, and -degradation. This tightly cont-
rolled network loses its equilibrium with age. To counter protein
homeostasis imbalances, organisms have evolved a set of cellu-
lar signaling cascades that are induced particularly upon stress,
such as heat or oxidative environments, to maintain protein
integrity. These so-called unfolded protein response (UPR) net-
works are specialized to the respective subcellular compartment
(mitochondrial or endoplasmic reticulum (ER) UPR, and the cy-
toplasmic heat shock response), but have in common that their
induction triggers the expression of chaperones. Chaperones
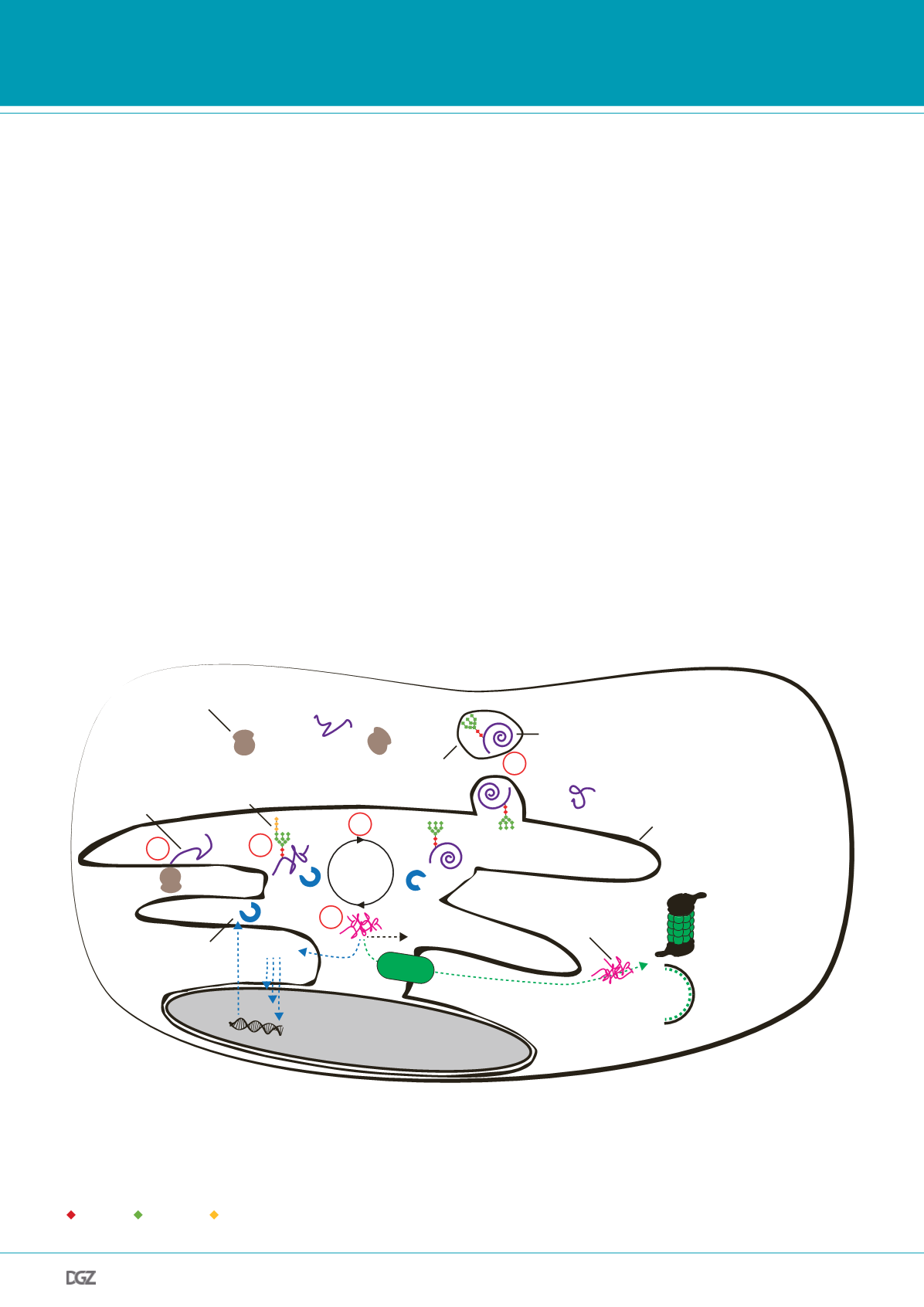
Figure 1. ER Protein Homeostasis:
Schematic representation of synthesis, folding, possible misfolding and degradation as well as transport of a secretory
protein: Secretory proteins enter the ER lumen co-translationally (1) and become N-glycosylated (2) before entering the calnexin/calreticulin folding cycle
(3). Proteins folded to their final conformation are shipped to the Golgi apparatus and the plasma membrane through vesicular transport (4). If chaperone-
assisted protein folding fails (5), misfolded proteins trigger the endoplasmic reticulum’s unfolded protein response (UPR
ER
), which in turn induces the
expression of chaperones. Misfolded proteins can be shuttled to the cytoplasm via the ER-associated degradation (ERAD) machinery to become substrates
for proteasomal or autophagic degradation. However, an overload of unfolded proteins can lead to toxic protein aggregation.
GlcNAc
Mannose
Glucose
RESEARCH NEWS
folding
cycle
nucleus
proteasomal
degradation
autophagy
ERAD
UPR
ER
chaperones
ribosome
nascent
polypeptide
vesicle
folded
protein
N-glycan
misfolded
protein
nucleus
1
2
3
4
5
aggregation
endoplasmic
reticulum


