12
Cell News 2/2015
RESEARCH NEWS
Hexosamine pathway
Glycolysis
Glucosamine-6-P
UDP-N-acetylglucosamine
(UDP-GlcNAc)
Glucose
Glucose-6-P
Fructose-6-P
Gln
Glu
GFAT-1
UPR
ER
AMPK/PKA
protein homeostasis
longevity
cardioprotection
N-glycosylation
mucine-type
O-glycosylation
O-GlcNAcylation
biopolymers
(chitin, GAGs)
proteasome activity
autophagy
ERAD
Pyruvate
others?
N-acetylglucosamine
(GlcNAc)
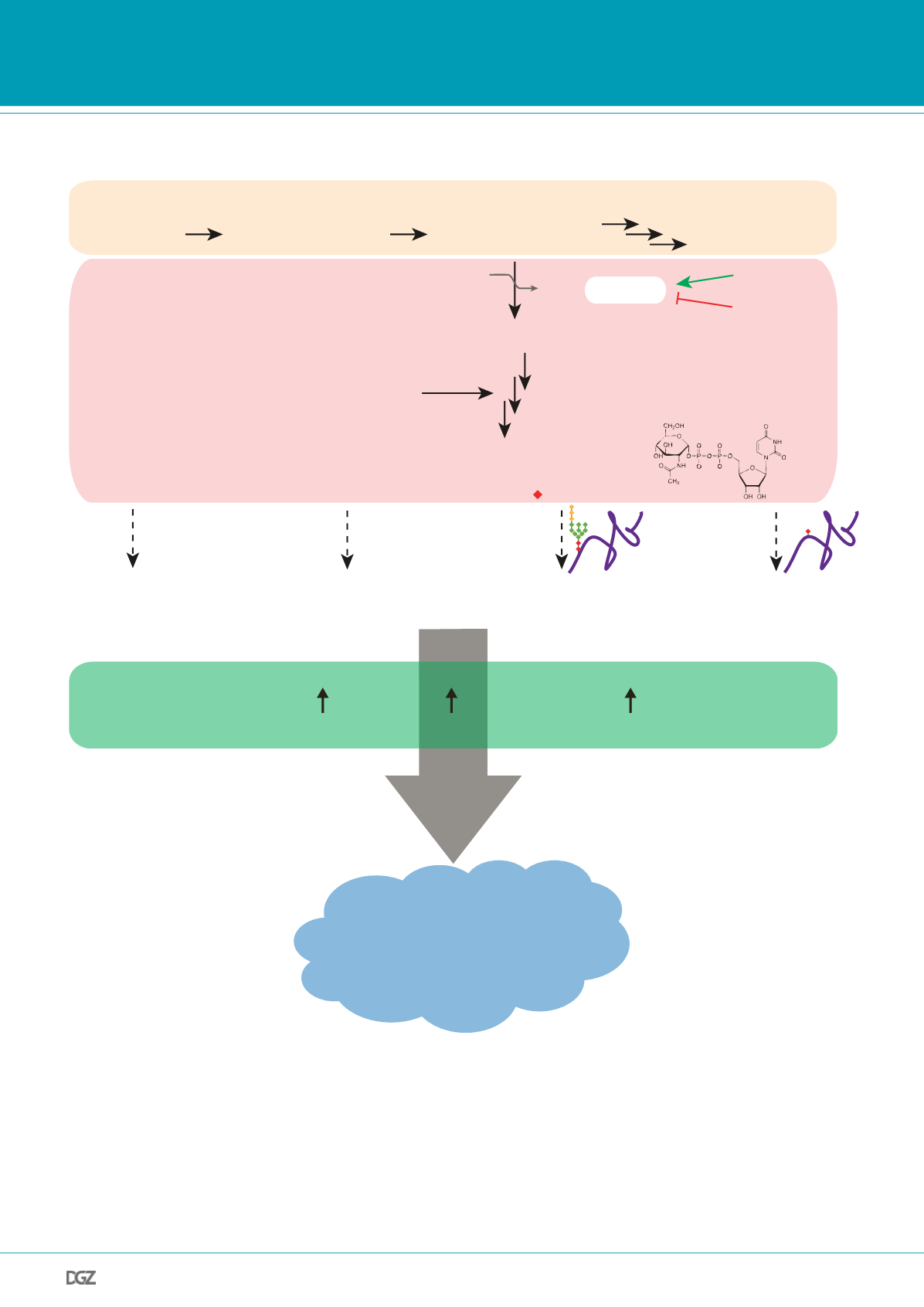
Figure 2: Hexosamine pathway flux affects protein homeostasis and longevity.
The hexosamine biosynthetic pathway (HP) shunts a fraction of
fructose-6-phosphate into the synthesis of the activated amino sugar UDP-N-acetylglucosamine (UDP-GlcNAc). UDP-GlcNAc serves as a building block
for N- and O-Glycan structures, can be added to proteins as a single moiety, and is also a component of biopolymers such as chitin or glycosaminoglycans
(GAGs). Activating the HP through gain-of-function mutation of the rate-limiting enzyme glutamine-fructose-6-phosphate aminotransferase (GFAT-1) or
by supplementing N-acetylglucosamine (GlcNAc) induces protein quality control mechanisms (proteasome activity, ER-associated degradation (ERAD), and
autophagy), resulting in increased lifespan and reduced proteotoxicity in the nematode
C. elegans
. In mammals, the ER unfolded protein response (UPR
ER
)
was shown to directly induce GFAT-1 expression, which exerts cardioprotective effects through increased O-GlcNAcylation. However, the mechanistic link
between HP activation and improved protein quality control remains entirely elusive.


