Cell News 3/2013
15
RESEARCH NEWS
such as apoptosis and cell differentiation (Balasubramanian and
Schroit, 2003). Perturbation of lipid asymmetry in several ty-
pes of cells was observed in the context of some pathological
conditions, e.g. tumors, diabetes and bleeding disorders (Fadeel
and Xue, 2009). Furthermore, the uneven distribution of lipid
species between the leaflets of a membrane bilayer determines
its physical properties, such as permeability, negative surface
charge (of the inner leaflet) and local curvature/shape (Rothman
and Lenard, 1977; Holzer et al., 2010). Membrane curvature in
particular is strongly connected to the intrinsic monolayer cur-
vature of specific lipids: a lipid with e.g. a small polar head has
a negative intrinsic curvature and will tend to bend a monolay-
er inwards. In this context, we have shown that enrichment of
ceramide (i.e. a sphingolipid with negative intrinsic curvature)
localized in one leaflet of giant unilamellar vesicles (GUVs) led
to spontaneous budding of small vesicles towards the lumen of
the GUV, as shown in Fig.1. This observation provided insights
into the molecular basis for a lipid-mediated ESCRT-indepen-
dent mechanism of membrane and cargo sorting in endosomes
(Trajkovic et al., 2008).
Transversal lipid asymmetry is also associated with the lateral
organization of lipids and proteins of the PM into domains.
Although still object of debate, the existence of nanoscale,
cholesterol-rich dynamic protein-lipid domains (sometimes
called “rafts”) is strongly supported by recent technical advan-
ces (Simons and Gerl, 2010). Such ordered assemblies of lipids
and proteins have been extensively studied in the last 20 years
in connection with a variety of biological processes including
immune response, cell-cell communication, viral infections and
membrane trafficking (Simons and Ikonen, 1997; Veit and Thaa,
2011). Of interest, according to the extensive experimental data
provided by biophysical model system studies, lipid mixtures mi-
micking the outer leaflet of the PM can give rise to raft-like or-
dered domains. On the other hand, lipid mixtures corresponding
to typical inner leaflet compositions form membranes that are
homogeneous (Wang and Silvius, 2001). Therefore, in the frame-
work of the raft model, the physical state and properties of the
inner leaflet of the PM are still not well understood.
Interaction between membrane leaflets:
role in trans-membrane coupling
Signal transduction across the PM requires the passage of infor-
mation from outside a cell to its inside and is usually triggered
by clustering or activation of receptors in the outer leaflet, i.e.
on the extracellular side (Simons and Gerl, 2010). It is known
that concerted reorganization of e.g. GPI-anchored receptors in
the outer leaflet of the PM and lipid-anchored signaling mole-
cules in the inner leaflet, such as Src-family kinases, is involved
in signal transduction (Stefanova et al., 1991). While clustering
and lateral organization of outer leaflet components might be
mediated by the presence of ordered domains, it is unclear how
lipid-anchored signaling molecules are recruited to the same
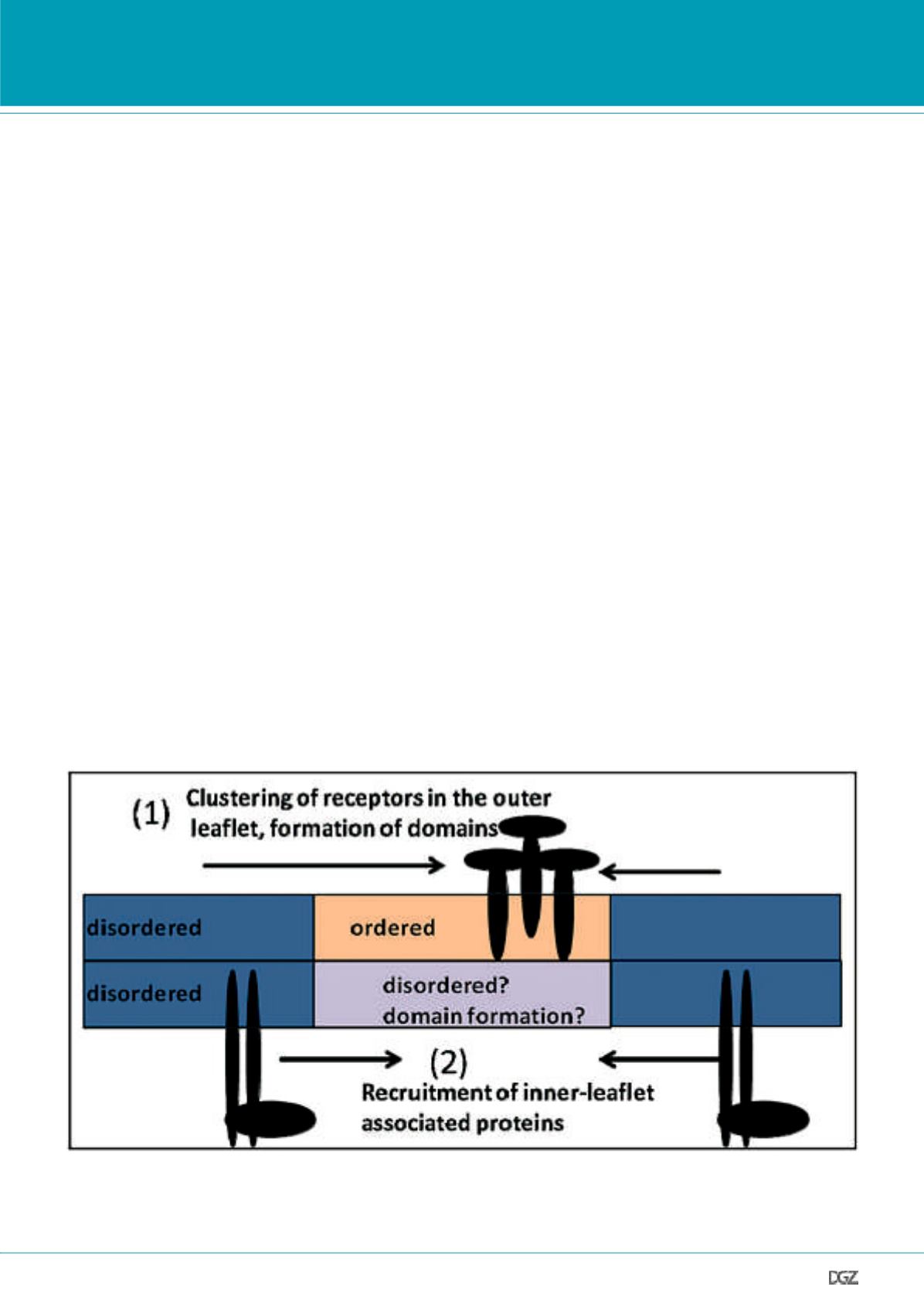
position and clustered (since the inner leaflet does not support
formation of lipid protein domains, see Fig.2). This raises the
general question of how leaflets´ physical properties are coup-
led, and what are the functional consequences of inter-leaflet
coupling.
In a similar context, the physical state of the cytosolic side of
PM domains might affect the lateral organization of trans-
membrane receptors. This is of great importance in the field of
Figure 2. Membrane reorganization during signaling initiation:
1) Clustering of trans-membrane proteins or outer leaflet-associated receptors causes formation of stable ordered domains (pink). 2) Inner leaflet-associ-
ated kinases are recruited to the same location in order to continue the signaling cascade. The physical state and properties of the cytoplasmic leaflet of
the PM (especially in correspondence with ordered raft-like domains in the outer leaflet) is still not understood.


