Cell News 3/2013
17
RESEARCH NEWS
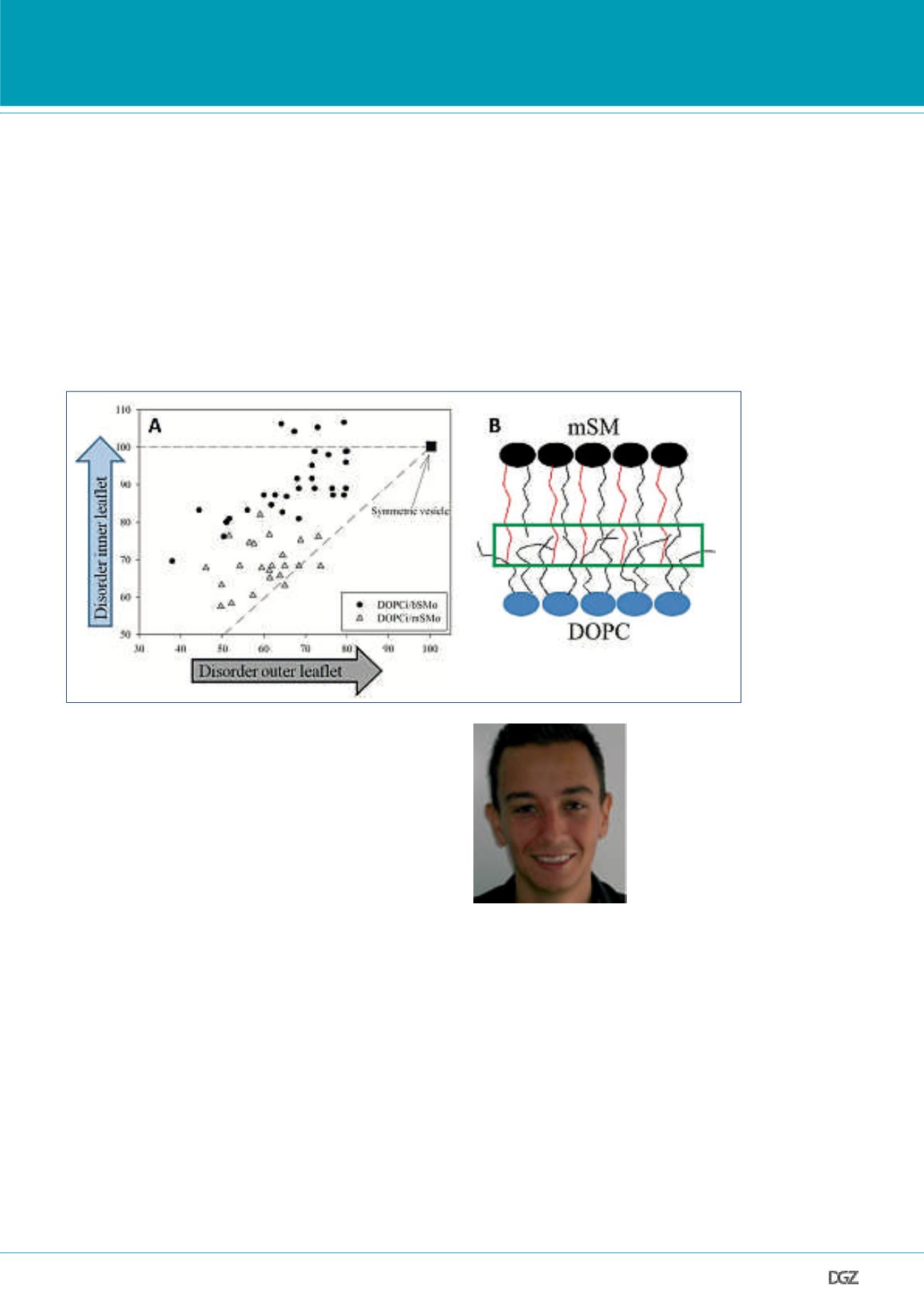
Figure 4. Coupling of physical properties (lipid diffusion) between inner and outer leaflet depends on lipid composition:
A) Asymmetric GUVs were prepared with different lipid compositions. Here, as an example, two compositions are shown: dioleoylphosphatidylcholine in the
inner leaflet and, in the outer leaflet, either SM from porcine brain (DOPCi/bSMo) or SM from bovine milk (DOPCi/mSMo). The main difference between mSM
and bSM is the length of their acyl chain: the long acyl chain of mSM can in fact penetrate (interdigitate) into the opposite (i.e. inner) leaflet. The graph in
this panel shows lipid diffusion (approximately related to local membrane order) in the inner leaflet as a function of the same parameter in the outer leaflet.
Each black circle refers to a single DOPCi/bSMo GUV. Grey triangles refer to single DOPCi/mSMo GUVs. The square in position (100,100) indicates the values
measured in a symmetric DOPC GUV, used as a reference. The measured points span a large window in the x-direction, since the observed vesicles contain
varying amounts of SM in the outer leaflet (i.e. SM in the outer leaflet induces ordering of the outer leaflet in a concentration-dependent fashion). It appears
that, in correspondence with a given state of the outer leaflet, the inner leaflet of asymmetric GUVs containing interdigitating mSM in the outer leaflet is
more ordered compared to GUVs containing non-interdigitating bSM in the outer leaflet. In other words, the inner leaflet of DOPCi/mSMo GUVs mirrors more
closely (i.e. is more coupled) to the outer leaflet, compared to the situation observed in DOPCi/bSMo GUVs. The dash-dot line has unitary slope (i.e. maximum
coupling); the dashed line has null slope (i.e. zero coupling). B) Schematic model of asymmetric bilayer with inner leaflet composed of DOPC (blue) and outer
leaflet composed of mSM (black). The green rectangle indicates the region near the bilayer mid-plane, where acyl chains from opposing leaflets might interact
thus inducing inter-leaflet coupling. The red lines represent the long interdigitating acyl chains of mSM. Adapted from (Chiantia and London, 2012).
Chiantia S, London E (2012). Acyl chain length and saturation modulate interleaflet coupling
in asymmetric bilayers: effects on dynamics and structural order. Biophys J 103, 2311-2319.
Chiantia S, Schwille P, Klymchenko AS, London E (2011). Asymmetric GUVs prepared by Mbe-
taCD-mediated lipid exchange: an FCS study. Biophys J 100, L1-3.
Devaux PF (1991). Static and dynamic lipid asymmetry in cell membranes. Biochemistry 30,
1163-1173.
Fadeel B, Xue D (2009). The ins and outs of phospholipid asymmetry in the plasma membrane:
roles in health and disease. Crit Rev Biochem Mol Biol 44, 264-277.
Garg S, Ruhe J, Ludtke K, Jordan R, Naumann CA (2007). Domain registration in raft-mimi-
cking lipid mixtures studied using polymer-tethered lipid bilayers. Biophys J 92, 1263-1270.
Holzer M, Momm J, Schubert R (2010). Lipid transfer mediated by a recombinant pro-sterol
carrier protein 2 for the accurate preparation of asymmetrical membrane vesicles requires a
narrow vesicle size distribution: a free-flow electrophoresis study. Langmuir 26, 4142-4151.
Hussain NF, Siegel AP, Ge Y, Jordan R, Naumann CA (2013). Bilayer asymmetry influences
integrin sequestering in raft-mimicking lipid mixtures. Biophys J 104, 2212-2221.
Iwabuchi K, Nakayama H, Iwahara C, Takamori K (2010). Significance of glycosphingolipid
fatty acid chain length on membrane microdomain-mediated signal transduction. FEBS Lett
584, 1642-1652.
Pautot S, Frisken BJ, Weitz DA (2003). Engineering asymmetric vesicles. Proc Natl Acad Sci U
S A 100, 10718-10721.
Rothman JE, Lenard J (1977). Membrane asymmetry. Science 195, 743-753.
Simons K, Gerl MJ (2010). Revitalizing membrane rafts: new tools and insights. Nat Rev Mol
Cell Biol 11, 688-699.
Simons K, Ikonen E (1997). Functional rafts in cell membranes. Nature 387, 569-572.
Son M, London E (2013). The dependence of lipid asymmetry upon phosphatidylcholine acyl
chain structure. J Lipid Res 54, 223-231.
Stefanova I, Horejsi V, Ansotegui IJ, Knapp W, Stockinger H (1991). GPI-anchored cell-surface
molecules complexed to protein tyrosine kinases. Science 254, 1016-1019.
Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brugger B, Simons
M (2008). Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Sci-
ence 319, 1244-1247.
Veit M, Thaa B (2011). Association of influenza virus proteins with membrane rafts. Adv Virol
2011, 370606.
Wan C, Kiessling V, Tamm LK (2008). Coupling of cholesterol-rich lipid phases in asymmetric
bilayers. Biochemistry 47, 2190-2198.
Wang TY, Silvius JR (2001). Cholesterol does not induce segregation of liquid-ordered domains
in bilayers modeling the inner leaflet of the plasma membrane. Biophys J 81, 2762-2773.
Salvo Chiantia studied Physics in
the University of Palermo where he
graduated in 2003. In 2008, he re-
ceived his PhD in Physics under the
supervision of Prof. Schwille in the
TU-Dresden, working on a combi-
nation of AFM and single-molecu-
le fluorescence for the study of li-
pid membranes. Between 2009
and 2012, he worked as a research
scientist in the group of Prof. Lon-
don (Stony Brook University, NY) where he developed a method
for the production of asymmetric giant lipid vesicles (GUVs). At
the present, he is holding a PostDoc position in the Department
of Biology / A. Herrmann’s group at the HU Berlin, studying in-
fluenza virus assembly.


