Cell News 3/2013
16
RESEARCH NEWS
membrane biophysics in general, because the origin of mem-
brane protein affinity for lipid rafts remains to date unknown
(see Fig.3).
Despite the strong biological relevance of these questions, a
systematic biophysical investigation could not be performed
until now, due to the absence of physical models of PM asym-
metry. Nearly all membrane biophysical studies in the last de-
cades have been in fact performed on symmetric lipid bilayers
and, therefore, might have provided only partial insights into
PM structure and organization.
Asymmetric lipid bilayers as advanced models
of the plasma membrane
In order to fill this significant knowledge gap, we and other
groups have recently developed new and advanced model mem-
brane systems that can effectively mirror PM compositional
asymmetry. Asymmetric supported lipid bilayers have been pre-
pared using vesicle fusion, sequential depositing of monolayers
or a combination of the two approaches (Garg et al., 2007; Wan
et al., 2008). However, such systems might be limited in terms
of what kind of experiments can be carried out and are sub-
jected to the influence of the solid support. Leaflet-by-leaflet
assembly of free-standing membranes has also been reported
(Pautot et al., 2003). Nevertheless, it is a concern that these
bilayers may incorporate the organic solvents used (e.g. mineral
oil). Therefore, we have developed a method based on methyl-
-cyclodextrin mediated outer leaflet exchange, in order to pro-
duce asymmetric vesicles with sizes ranging from 100 nm (small
or large unilamellar vesicles (SUVs or LUVs)) to 100 µm (GUVs)
(Cheng et al., 2009; Cheng and London, 2011; Chiantia et al.,
2011). This approach is fast, has a high yield, makes use of con-
ventional vesicle preparation techniques and can be used with a
wide variety of lipids (Son and London, 2013). Furthermore, this
methodology is fully compatible with the most common protein
reconstitution protocols.
In order to study inter-leaflet coupling, we have produced
asymmetric GUVs with a biologically relevant composition, i.e.
with an outer leaflet rich in SM and an inner leaflet rich in phos-
phatidylcholine (Chiantia and London, 2012). Specific molecular
mechanisms involved in inter-leaflet coupling have been sug-
gested to include cholesterol trans-bilayer dynamics, electro-
static interactions and lipid acyl chain interdigitation. By using
fluorescence correlation spectroscopy (FCS), we monitored lipid
diffusion (i.e. membrane order) separately in each leaflet as a
function of lipid composition. We were able to show that the
presence of SM from bovine milk (i.e. an interdigitating lipid
with acyl chain significantly longer than the sphingoid back-
bone) in the outer leaflet induced an ordering effect also in the
inner leaflet (see Fig.3). This finding is particularly interesting
in light of the fact that the presence of interdigitating sphin-
golipids is necessary for a correct trans-membrane signaling in
neutrophils (Iwabuchi et al., 2010). By analyzing different lipid
compositions, we could conclude that inter-leaflet coupling de-
pends on both length and saturation of lipid acyl chains (Chian-
tia and London, 2012). The molecular mechanism likely involves
van der Waals interactions between the terminal portions of the
acyl chains of facing lipid molecules occurring at and near the
bilayer midplane (Fig.3B).
In conclusion, the above-mentioned advanced biophysical mo-
dels of the PM might allow a better understanding of lipid-
mediated inter-leaflet coupling and signal transduction through
the PM in the future, also in connection with the role of ste-
rols and membrane proteins. Furthermore, asymmetric bilayers
could clarify the role of PM compositional asymmetry in de-
termining its three-dimensional structure (i.e. membrane cur-
vature) and protein partition into lipid domains. The relationship
between PM asymmetry and receptor lateral organization has in
fact only very recently started to be addressed systematically in
controlled systems (Hussain et al., 2013).
References
Balasubramanian K, Schroit AJ (2003). Aminophospholipid asymmetry: A matter of life and
death. Annu Rev Physiol 65, 701-734.
Bretscher MS (1972). Asymmetrical lipid bilayer structure for biological membranes. Nat New
Biol 236, 11-12.
Cheng HT, London E (2011). Preparation and properties of asymmetric large unilamellar ve-
sicles: interleaflet coupling in asymmetric vesicles is dependent on temperature but not cur-
vature. Biophys J 100, 2671-2678.
Cheng HT, Megha, London E (2009). Preparation and properties of asymmetric vesicles that
mimic cell membranes: effect upon lipid raft formation and transmembrane helix orientation.
J Biol Chem 284, 6079-6092.
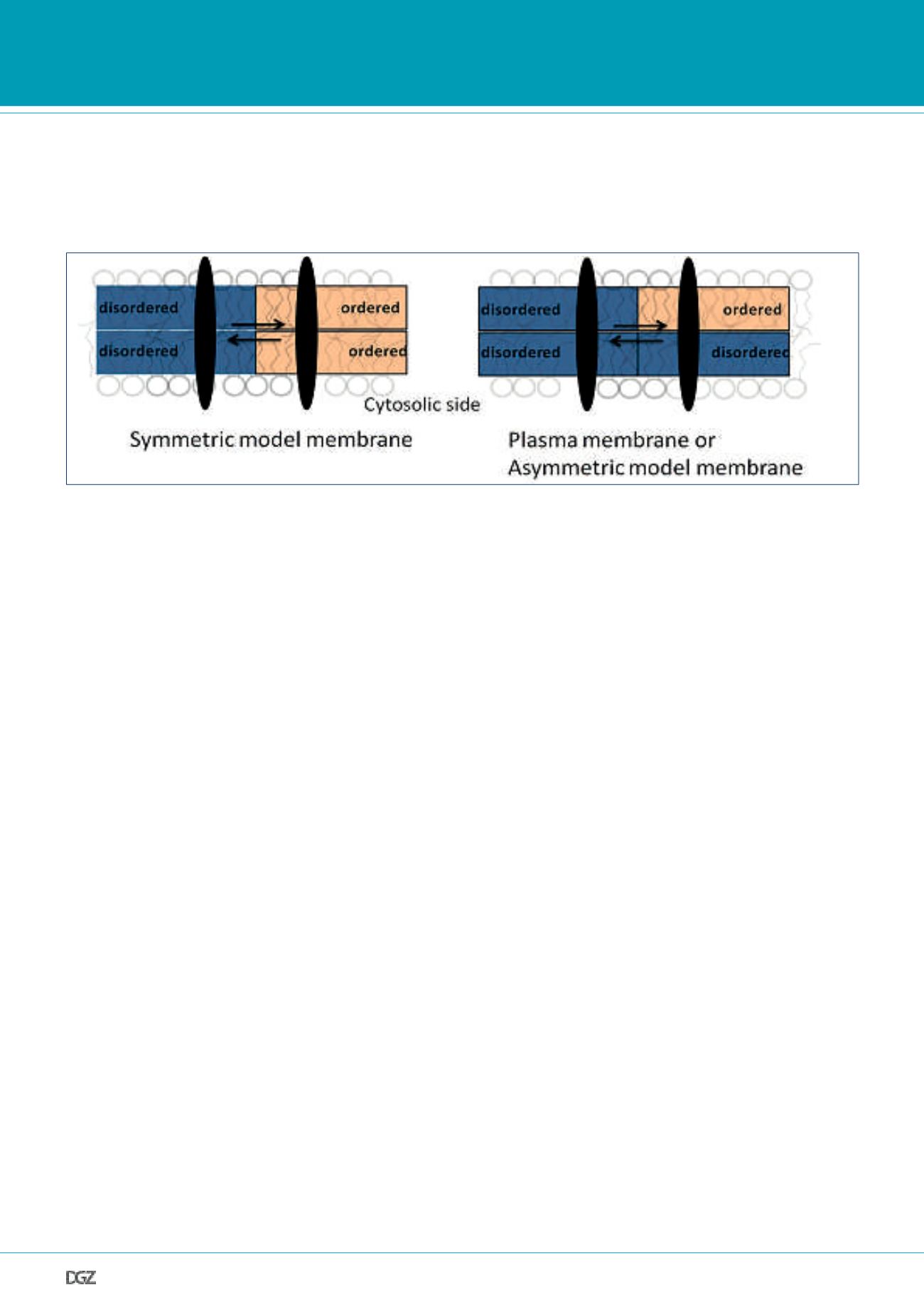
Figure 3. Partitioning of a trans-membrane protein between ordered and disordered domains:
In symmetric model membranes, the ordered domains are rich in ordering lipids (e.g. SM and cholesterol, pink) in both leaflets. In the PM (modeled by
asymmetric bilayers), the ordered domains are rich in ordering lipids on the extracellular side, but rich in disordering lipids (e.g. unsaturated phospholipids,
blue) on the cytosolic side. Since protein partitioning is strongly affected by lipid order, membrane asymmetry might play an important role in this process.


