Cell News 3/2013
17
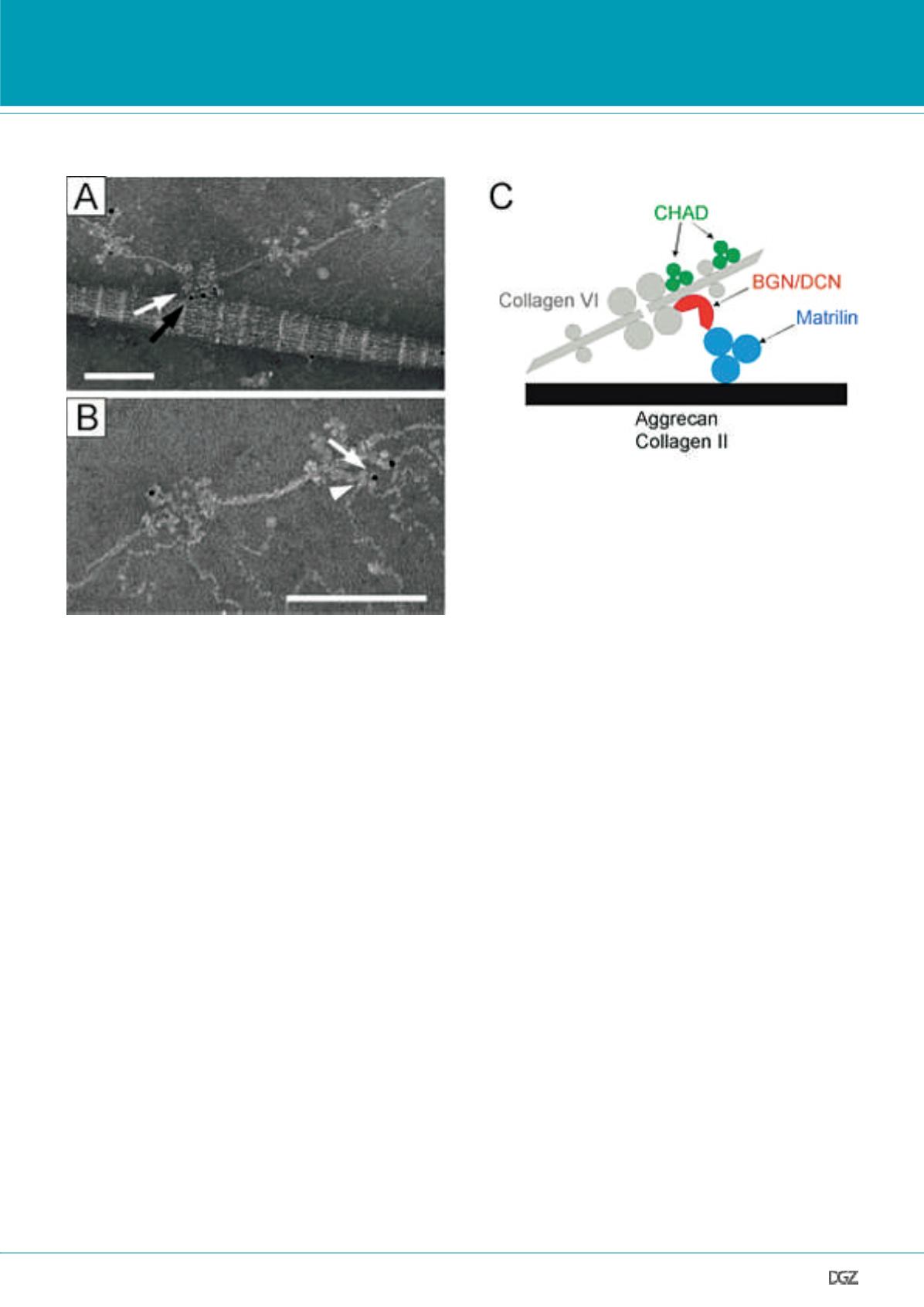
Figure 2. Complexes of matrilin-1 and biglycan or decorin connect collagen VI microfibrils to both collagen II and aggrecan in cartilage.
By using electron microscopy and gold labelled antibodies, components of collagen II (A) and aggrecan (B) networks were found connected to collagen VI
microfibrils via the matrilin/small leucine-rich repeat proteoglycan complex. (A) Double staining with gold particles of different sizes occasionally located
complexes of biglycan (white arrow, small gold) and matrilin-1 (black arrow, large gold) between collagen VI microfibrils and striated collagen II fibrils. (B)
Arrowhead points to matrilin-1 consisiting of three globular domains. The gold labelled antibody detects matrilin-1 (arrow). These interactions are summa-
rized in a schematic model (C). BGN, biglycan; DCN, decorin; CHAD, chondroadherin. Modified from Wiberg et al., 2003.
RESEARCH NEWS
and immunological detection of molecular constituents. Using
this powerful methodology, it was shown that small leucine-rich
proteoglycans and matrilins act as linkage between collagen VI
microfibrils and aggrecan, or alternatively collagen II (Fig. 2). The
analysis of protein-protein interactions contributed significantly
to the model of the supramolecular assembly in the cartilage
extracellular matrix.
Interestingly, the adaptor proteins matrilin-3 and COMP do not
only share several binding partners but also interact directly with
each other (Mann et al., 2004). Taken together, this has led to
the assumption that COMP, matrilin-3 and collagen IX as well as
the small leucine rich proteoglycans decorin and biglycan, when
assembled in defined complexes, interconnect the fibrillar colla-
gen network and the aggrecan gel in cartilage. However, it is still
unclear if and which perifibrillar proteins are essential for the
proper formation of such suprastructures.
As mentioned above, perifibrillar proteins are integrated direct-
ly or indirectly into the collagenous bodies of collagen fibrils.
It seems as if the function of these proteins goes beyond sim-
ply connecting different structures. Several studies, including
our own, have shown that perifibrillar proteins are involved in
the regulation of the collagen fibril diameter (Blumbach et al.,
2009; Otten et al., 2010; Hagg et al., 1998; Kalamajski & Old-
berg, 2010). It could be shown that COMP first binds to and then
promotes the early assembly of collagen molecules into fibrils
(Halasz et al., 2007). To get further insight into the role of COMP
during fibril formation the effect of disease causing mutant va-
riants on fibrillogenesis was analyzed. Turbidity measurements
and analysis by electron microscopy confirmed the catalyst
function, but also revealed that a chondrodysplasia-causing mu-
tant COMP (p.H587R) induced collagen fibril aggregation and
disorganisation rather than promoting the formation of a typi-
cally banded fibril structure (Hansen et al., 2011; Fig. 3). These
experiments showed that the analysis of mutant variants could
be helpful for the understanding of the physiological function
of a protein. Similar experiments were performed to analyse the
disease causing mechanisms of a matrilin-3 mutation that has
been linked to osteoarthritis (OA). Even if the collagen affinity
was not affected, the formation of wider cartilage collagen fib-
rils was observed in the presence of the mutant variant (Otten et
al., 2010). Certainly, potential functions of perifibrillar proteins
deduced from such in vitro studies have to be confirmed in vivo.
Human diseases
Mutations in perifibrillar proteins, including COMP, collagen IX
and matrilin-3 cause a broad spectrum of skeletal conditions
(Warman et al., 2011). Two of them, pseudoachondroplasia
(PSACH) and multiple epiphyseal dysplasia (MED) are autosomal


