Cell News 3/2013
12
RESEARCH NEWS
in the regulation of e.g. innate immunity versus polarity. On the
other hand, both isoforms are necessary to polarize T-cells in
vivo and couple this to an effective Th2-response (Martin et
al., 2005; Yang et al., 2009). Thus, at present it is unclear if
both aPKCs are functionally redundant or have separate func-
tions in the regulation of polarity, metabolism and immunity.
Although both aPKC isoforms are expressed in the skin, real time
PCR analysis revealed that aPKC
λ
is expressed around 10-fold
more strongly in mouse epidermis. In addition, whereas aPKC
ζ
is confined to the basal layer of the epidermis, aPKC
λ
is strongly
enriched at sites of intercellular junctions in the suprabasal lay-
er of the epidermis, suggesting that this isoform may regulate
epidermal polarity and barrier function.
aPKC and the regulation of barrier function in
stratifying epithelia
Perhaps the best characterized example of cell polarity is apico-
basolateral polarity, also known as epithelial polarity, in which
simple epithelia such as the intestine establish two different
membrane domains, the apical and basolateral domains that are
separated by the apical intercellular junctional complex con-
sisting of tight junctions, adherens junctions and desmosomes
(Roignot et al., 2013). Apico-basolateral polarity is important
for barrier function, vectorial transport and sensory and signal
perception. The stratifying epidermis is not a classically po-
larized epithelium like the intestine, in which tight junctions
separate basolateral and apical membrane proteins and lipids
(Fig.1A). Instead, the epidermis establishes polarity along the
basal to apical axis of the tissue, with the stratum granulo-
sum forming the viable apical boundary (Fig.1B). The formati-
on of the stratum corneum depends on the fusion of lamellar
bodies, specialized secretory granules containing enzymes and
lipids necessary to build up the stratum corneum with plasma
membranes at the transition between stratum granulosum and
corneum layers. As in simple epithelia, tight junctions in the
stratum granulosum may thus have a fence function that may
be necessary for “apical” targeting of these lipid vesicles directly
towards the stratum corneum.
From C. elegans to humans, the formation and maintenance of
intercellular junctions and apical membrane domain identity in
simple epithelia is tightly linked to the Par3/Par6/aPKC com-
plex (Nelson, 2003; Goldstein and Macara, 2007). It is thus well
possible that the positioning of functional tight junctions and
the formation/maintenance of the apical domain in stratifying
epithelia would also require the activity of the apical Par3/Par6/
aPKC complex. Par3/Par6/aPKC coordinates simple epithelial
polarity through mutual inhibitory and activating interactions
with other polarity complexes, such as the LGL/Scribble and the
Crumbs complex, within the same cell (Fig. 1A). The mechanisms
that regulate the formation of stratifying apico-basolateral tis-
sue polarity are largely unknown. If similar mechanisms are in
place as in simple epithelia then the mutual antagonistic ac-
tions of polarity complexes have to be established over several
cell layers. A relatively simple system could consist of counter-
gradients of mutually inhibiting complexes over the basal-apical
axis of the epidermis (Fig.1B). Interestingly, as in simple epithe-
lia, both Rac and Par3 are necessary for tight junctional barrier
function in stratifying keratinocytes (Mertens et al., 2005; Iden
et al., 2012), suggesting a similar mechanism as in simple epi-
thelia at least for formation of functional tight junctions.
To examine whether aPKC regulates epidermal barrier func-
tion we exogenously expressed aPKC in primary keratinocytes
and observed that whereas wt aPKC would enhance epidermal
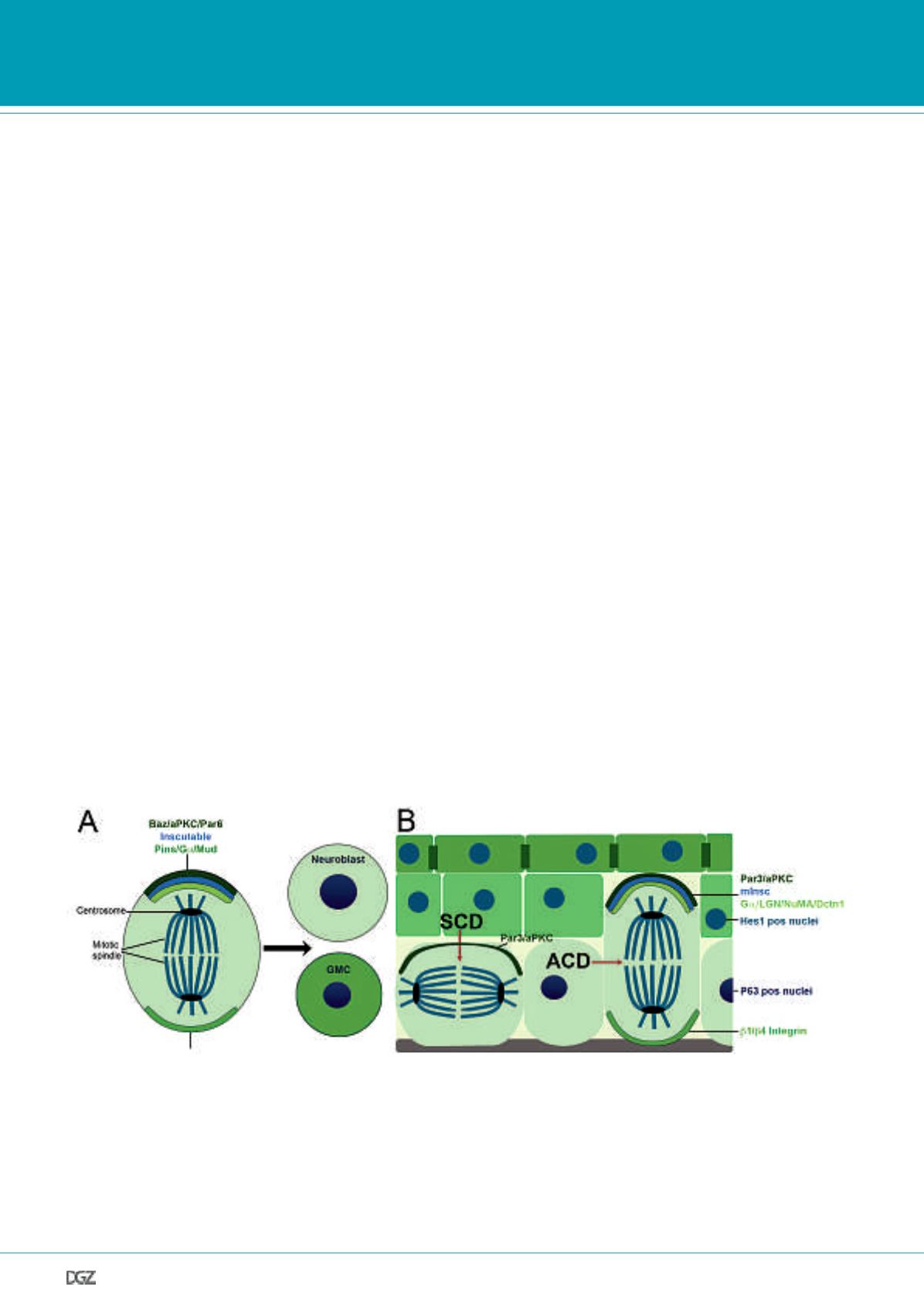
Figure 2. Mechanisms of asymmetric cell division.
Schematic overview of asymmetric localization of polarity proteins and spindle orientation regulators during asymmetric cell division in Drosophila (panel
A) and in the interfollicular epidermis (panel B), illustrating that similar molecular mediators are involved in the establishment of asymmetric cell divisions
of neuroblasts and keratinocytes. (A) Asymmetric cell division (ACD) in Drosophila neuroblasts. The apical aPKC-Baz-Par6 complex is connected to the
Pins-G
a
1-MUD complex via Inscuteable. This complex directs the asymmetric basal localization of the cell fate determinants Numb, Brat and Prospero.
GMC, Ganglion mother cell. (B) ACD in the developing IFE. ACD contribute to stratification by producing one basal, proliferating cell (light green) and
one suprabasal cell (dark green), whereas symmetric cell divisions (SCD) result in two daughter cells residing in the basal layer. aPKC-Par3, mInsc and
G
a
1-LGN-NuMA-Dcnt1 localize to one side of the dividing cell and are important for the establishment of epidermal ACD, as reported for their Drosophila
homologues in neuroblast ACD. Suprabasal activity of the Notch signaling pathway (indicated by nuclei positive for Hes1, a well known Notch target) are
crucial for the regulation of this process.


