Cell News 3/2013
13
RESEARCH NEWS
barrier function overexpression of dominant negative aPKC
mutants interfered with TJ function (Helfrich et al., 2007). In
collaboration with Michael Leitges (Biotechnology Centre of
Oslo, University of Oslo) we deleted aPKC
λ
in mouse epidermis
using the Cre-LoxP system. Isolated keratinocytes from these
mice showed a reduced TER that in vivo was associated with
cytoskeletal changes, altered differentiation and proliferation
accompanied by inflammation, similar to what is observed in
very common skin barrier associated diseases such as ichtyosis
or psoriasis. Together, these results suggest a specific function
of aPKC
λ
in skin barrier regulation. However, initial characteri-
zation of mice in which both aPKCs are absent showed a much
more severe morphogenetic and barrier dysfunction phenotype,
indicating specific and overlapping functions of the two aPKCs
in the epidermis. Thus, aPKCs integrate cell polarity, nutrient si-
gnaling and regulation of innate immunity to coordinate tissue
architecture and barrier function.
The role of aPKC in mammalian cell division orientati-
on and cell fate
In lower organisms aPKC controls cell fate and asymmetric cell
division (ACD) (Lee et al., 2006; Knoblich, 2010), resulting in two
daughter cells with differential fate. In Drosophila neuroblasts,
the initial polarization cue comes from the apical enrichment of
the aPKC/Par complex (Fig.2A). This apical distribution is essen-
tial for asymmetric localization of cell fate determinants, which
is coupled to spindle orientation by binding to the adaptor pro-
tein Inscuteable (Insc). Insc then recruits a protein complex con-
sisting of the heterotrimeric G protein
α
1-subunit (G
α
1), PINS
and MUD, which provides attachment sites for astral microtu-
bules (Knoblich, 2010).
Whether oriented division regulates adult tissue homeostasis or
if aPKCs determine division orientation and cell fate in mam-
mals is not known. Whereas in vitro and ex vivo studies indicate
an important role for aPKC
λ
and/or aPKC
ζ
, in spindle orientation
and cell fate (Dard et al., 2009; Hao et al., 2010; Durgan et
al., 2011), in vivo inactivation in the hematopoetic or neuronal
systems indicate no essential role for aPKCs in these processes
(Imai et al., 2006; Sengupta et al., 2011).
The epidermis contains different progenitor cell populations and
at least in the IFE it was shown that ACD at least in part drives
differentiation (Lechler and Fuchs, 2005; Niessen et al., 2012).
This tissue thus provides an excellent model system to address
the role of balancing SCD and ACD and its regulators in tissue
homeostasis, differentiation and cell fate determination. As in
Drosophila neuroblasts, Par3 and aPKC show an apical distri-
bution in murine epidermis that is independent of cell division
(Lechler and Fuchs, 2005). This apical polarity might have been
Quiescent stem cells
Proliferation
Ageing
ACD
ACD
SCD
Self renewal
Self renewal
Ctr
P 2 1
P 3 3
P 4 7
P 5 8
P10 0
0
0
0.5
1.0
1.5
2.0
Ctr
fo ld change
*
**
**
**
% "
6
+ /
CD34
+
cells
aPKC!
epi-/-
snoisivi
d
f
o
noi
tcar
f
Bulge
snoisivi
d
f
o
no
itcar
f
Ctr
aPKC
!
epi-/-
embryonic
sim
red
i
p
e
ProgenyLgr5
+
;aPKCλ
-
cells
Labeling
Lgr5
+
;aPKCλ
+
cells
telogen
anagen
hairgerm
Junctionalzone/
Infundibulum
Sebaceousgland
Bulge
Hairshaft
Bulb
ProgenyLgr5
+
;aPKCλ
+
cells
anagen
Ctr
aPKC!
Lgr5 -/-
ACD
SCD
aPKC/
γ
-tub/
DAPI
5 µm
A
B
C
D
E
F
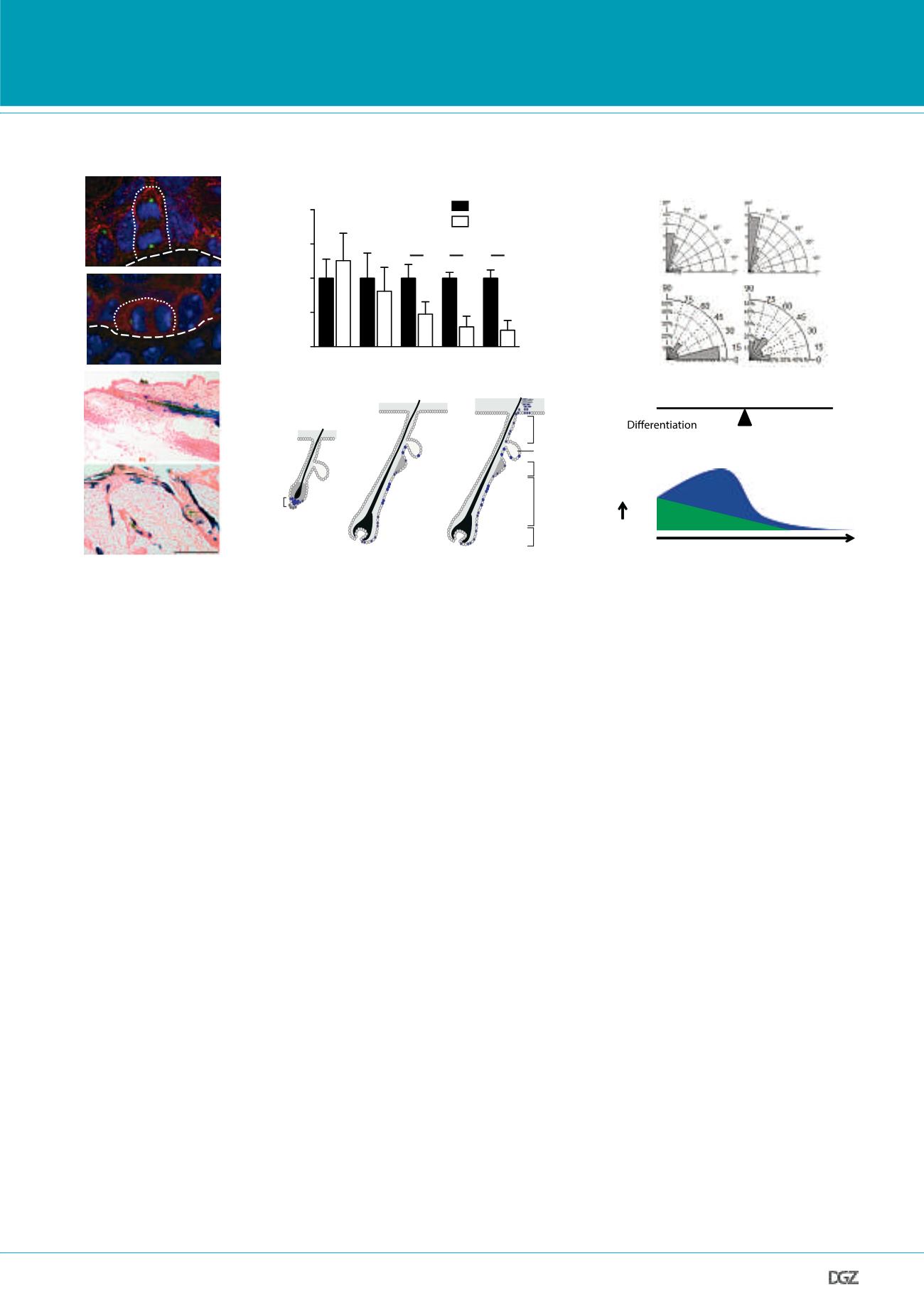
Figure 3. aPKC
λ
controls oriented cell division and cell fate in the mammalian epidermis.
(A) Apical localization of aPKC in asymmetric and symmetric divisions in the interfollicular epidermis. (B) Gradual loss of hair follicle bulge stem cells in
epidermal specific aPKC
λ
knockout mice. Quantification of FACS analysis of integrin
α
6+/CD34+ bulge hair follicle stem cells from epidermis at indica-
ted time points. (C) Spindle orientation plots reveal that epidermal loss of aPKC
λ
induces a shift towards more asymmetric divisions in the developing
interfollicular epidermis (embryonic epidermis) and in the bulge hair follicle stem cell compartmet in adults (bulge). (D and E) Loss of aPKC
λ
alters the fate
of lower bulge stem cells. Genetic lineage trace analysis reveal that aPKC
λ
-negative lower bulge stem cells do not only contribute to lower hair follicle
regeneration, as controls, but now fuel the upper hair follicle (junctional zone/infundibulum), sebaceous glands and interfollicular epidermis. (F) Model
proposing that a shift towards more asymmetric cell division promotes loss of quiescent hair follicle bulge stem cells that become more committed proge-
nitors that initially expand but as these also undergo increased asymmetric division, these cells also are depleted leading to increased differentiation and
premature skin aging.


