12
Cell News 1/2015
Perspective
tion from pointed ends, thus providing fresh G-actin molecules
to the actin pool (reviewed in (Mizuno, 2013)). Because cofilin
has emerged as a central player in actin filament turnover and
the generation of free barbed ends in various cell lines and or-
ganisms its activity needs to be tightly controlled. Several con-
trol mechanism such as the intracellular pH, phosphoinositides
and the phosphorylation state of serine 3 have been identified
(reviewed in (Mizuno, 2013)). Especially the phosphorylation-
dependent regulation of cofilin is well understood and involves
the balanced action of several kinases and phosphatases. Phos-
phorylation of cofilin at serine 3 by the LIM kinase (LIMK) fa-
mily (LIMK1 and LIMK2) and the related testicular protein (TES)
kinases turns off the actin-binding activity of cofilin and thus
leads to inactivation. On the other hand, dephosphorylation
by slingshot (SSH1, SSH2, SSH3) as well as chronophin phos-
phatases results in reactivation of the actin binding activity of
cofilin (reviewed in (Mizuno, 2013)). Accordingly, the level and
activity of cofilin kinases and phosphatases are tightly regu-
lated as well by a variety of proteins. Among those is the Rho
family of small GTPases. In humans, more than 20 Rho proteins
have been identified with RhoA, Rac1 and Cdc42 being the best
characterized members (Bos et al., 2007). Rho GTPases are key
regulators of the actin and microtubule cytoskeleton, thereby
controlling different steps of cell migration, adhesion and po-
larity, and vesicular trafficking (Hall, 2012). Rho protein activity
is tightly controlled in a spatial and temporal manner by three
classes of regulators: Firstly, guanine exchange factors (GEFs)
promote the exchange of bound GDP for GTP, leading to activa-
tion of the Rho GTPase and subsequent binding of downstream
effectors. Activated Rho GTPases are targeted to cell membra-
nes by their C-terminal prenyl groups serving as lipid anchors.
Secondly, GTPase activating proteins (GAPs) enhance the low
intrinsic GTPase function of the Rho proteins thereby leading to
their inactivation. Lastly, binding of guanine nucleotide dissoci-
ation inhibitors (GDIs) keeps Rho GTPases in the inactive state
by preventing the release of GDP or by masking the prenyl group
thereby sequestering Rho GTPases in the cytoplasm (Bos et al.,
2007).
Rho GTPases and the cofilin signaling network are connected on
multiple levels: For example, the Rho effector kinase ROCK di-
rectly phosphorylates and activates LIMK on a conserved threo-
nine residue in the activation loop of the kinase domain. In addi-
tion, the Cdc42 effector kinase MRCK
α
acts on both, LIMK1 and
2, whereas the Cdc42 and Rac effector kinases PAK1 and PAK4
exclusively phosphorylate and activate LIMK1 (Scott and Olson,
2007). Other key players in the cofilin signaling network are the
three members of the protein kinase D (PKD) family, PKD1, PKD2
and PKD3. The three isoforms are central regulators of vesicu-
lar trafficking but also directed cell migration and invasion by
controlling F-actin dynamics. PKD is activated downstream of
Rho but also Rac and, by direct phosphorylation, impacting its
substrates PAK4 and SSH1 in a positive and a negative manner,
respectively, the consequence of which is the inactivation of
cofilin (Olayioye et al., 2013). Because SSH1 has been recently
identified to be a central regulator of NOD1-mediated signaling
(Bielig et al., 2014) it is intriguing to speculate that PKD con-
tributes to innate immunity responses as well. This assumption
is supported by studies in
C. elegans
showing that animals who
have lost DKF-2, a
C. elegans
PKD, were hypersensitive to killing
by bacterial pathogens (Ren et al., 2009).
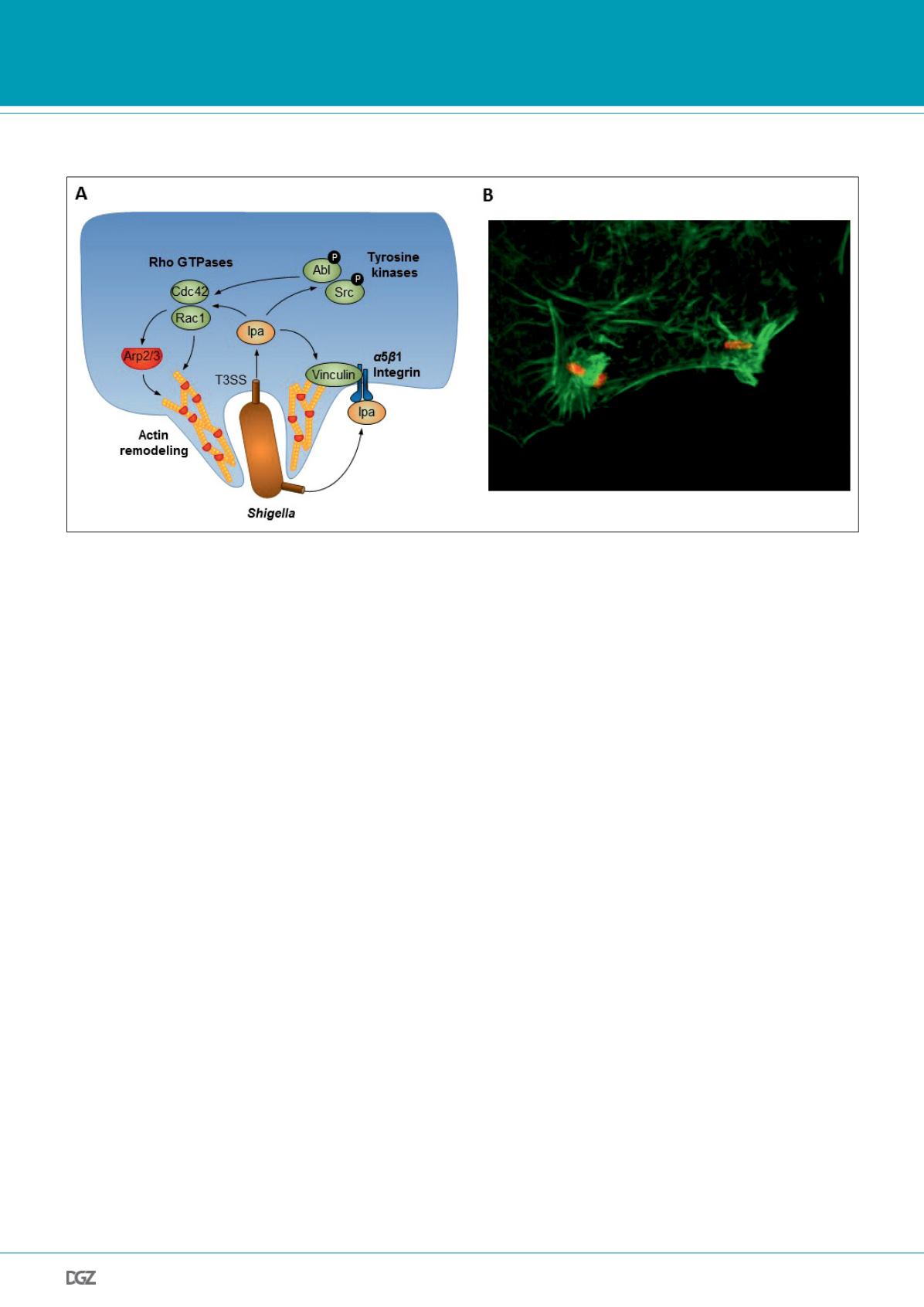
Figure 1:
(A) Effector mediated targeting of host cell signaling pathways regulating F-actin dynamics. The pathogen (e.g.
Shigella
) delivers invasion
plasmid antigen (Ipa) proteins that induce host cytoskeletal rearrangements on different levels and finally drive bacterial uptake. Ipa proteins activate the
Rho GTPases Rac1 and Cdc42 and promote F-actin remodeling via the Arp2/3 complex. Vinculin is either directly or indirectly targeted by Ipa proteins and
promotes F-actin reorganization. Tyrosine kinases Abl and Src are activated upon pathogen infection and lead to further F-actin rearrangements.
(B) Immunofluorescence micrograph showing F-actin (green) in a HeLa cell that is invaded by
S. flexneri
(red).


