14
Cell News 1/2015
Perspective
details of these interconnections still remain largely elusive. A
protein that was recently recognized to be able to react towards
bacterial induced perturbations of GTPase functions is NOD1.
NOD1 is a bona fide PRR and the best described function of
NOD1 is the sensing of bacterial peptidoglycan in the cystosol
that induces cell-autonomous innate immune responses, which
is of particular importance for immune responses towards ente-
roinvasive bacterial pathogens. These bacteria, such as
Salmo-
nella
and
Shigella
enter epithelial cells by induced uptake upon
contact to host cells and delivery of type III effectors to the
host cell cytoplasm that induce profound changes in the corti-
cal F-actin network (Figure 1). For invasion of
S. flexneri
, it was
reported that GEF-H1, a GEF for RhoA plays an important role in
this process. Moreover, NOD1 localizes at F-actin rich structures
at the cell cortex and at the site of bacterial invasion (Kufer et
al., 2008). Recent evidence now links GEF-H1 to NOD1-medi-
ated detection of the
Shigella
effector proteins (Fukazawa et
al., 2008). Notably, induction of inflammatory responses by this
pathway requires RhoA mediated activation of Rho-associated
protein kinases (ROCKs) (Fukazawa et al., 2008). This would sug-
gest that NOD1 can monitor small Rho GTPase activity in the
host cell and translates pathogen induced perturbations into
inflammatory responses by activation of NF-
κ
B downstream of
NOD1. Evidence for such a function of NOD1 is provided by a
study that recently showed that the Rho GEF
Salmonella
SopE
can activate NOD1 (Keestra et al., 2013). We recently identified
another key component of the F-actin regulatory network, the
phosphatase SSH1 as critical component of NOD1-mediated re-
sponses (Bielig et al., 2014). Our studies strongly suggest a role
of SSH1 in NOD1 signaling as knockdown of cofilin mimicked
the effect of SSH1 depletion (Bielig et al., 2014). Furthermore,
we showed that modulation of ROCK activity equally resulted in
altered NOD1-mediated inflammatory responses (Bielig et al.,
2014).
In contrast to data suggesting that NOD1 senses actin pertur-
bation by directly sensing Rho activity, our data rather argue
that NOD1 activation by MAMPs results in activation of cofilin,
which is a prerequisite of downstream signaling to inflammato-
ry pathways. This on the other hand would offer an opportunity
for the host to integrate changes in the F-actin network induced
by bacterial effector proteins into pro-inflammatory signaling.
More studies are needed to validate this hypothesis and to bring
forward the involved players. However strong support for such a
scenario comes from two very recent publications: NLRC4 was
shown to induce actin polymerization upon activation and this
process was found to be essential for downstream activation
of caspase-1 but also for containing intracellular pathogens,
as shown for
Salmonella
(Man et al., 2014). Moreover, cofilin
was recently found to be a key player in the integration of TLR-
mediated and B cell receptor (BCR) signals in B cells (Freeman
et al., 2015).
Conclusion
Many bacterial pathogens, including also non-invasive bacteria,
such as enteropathogenic
Escherichia coli
induce profound alte-
rations of the cortical F-actin network. We assume that sensing
of such perturbations of host cell F-actin could be involved in
most innate immune reactions induced by bacterial pathogens
(Figure 2) and suggest cofilin as a key player of these responses.
Future research will address if and how the members of the co-
filin signaling network are regulated upon pathogen invasion to
impinge on cofilin activity. This research will also help to answer
the critical question in the field that is still controversially dis-
cussed: Do pattern-recognition receptors sense changes in Rho
GTPase activity directly or integrate pathogen induced respon-
ses into innate immune responses by the use of F-actin as a hub
to link to inflammatory pathways?
References
Aepfelbacher, M., Roppenser, B., Hentschke, M., and Ruckdeschel, K. (2011). Activity modula-
tion of the bacterial Rho GAP YopE: an inspiration for the investigation of mammalian Rho
GAPs. European journal of cell biology 90, 951-954.
Akira, S., Uematsu, S., and Takeuchi, O. (2006). Pathogen recognition and innate immunity.
Cell 124, 783-801.
Aktories, K. (2011). Bacterial protein toxins that modify host regulatory GTPases. Nat Rev
Microbiol 9, 487-498.
Arbibe, L., Mira, J.P., Teusch, N., Kline, L., Guha, M., Mackman, N., Godowski, P.J., Ulevitch,
R.J., and Knaus, U.G. (2000). Toll-like receptor 2-mediated NF-kappa B activation requires a
Rac1-dependent pathway. Nat Immunol 1, 533-540.
Bamburg, J.R. (1999). Proteins of the ADF/cofilin family: essential regulators of actin dyna-
mics. Annual review of cell and developmental biology 15, 185-230.
Baxt, L.A., Garza-Mayers, A.C., and Goldberg, M.B. (2013). Bacterial subversion of host inna-
te immune pathways. Science 340, 697-701.
Bernstein, B.W., and Bamburg, J.R. (2010). ADF/cofilin: a functional node in cell biology.
Trends in cell biology 20, 187-195.
Bielig, H., Lautz, K., Braun, P.R., Menning, M., Machuy, N., Brugmann, C., Barisic, S., Eisler,
S.A., Andree, M., Zurek, B., Kashkar, H., Sansonetti, P.J., Hausser, A., Meyer, T.F., and Kufer, T.A.
(2014). The Cofilin Phosphatase Slingshot Homolog 1 (SSH1) Links NOD1 Signaling to Actin
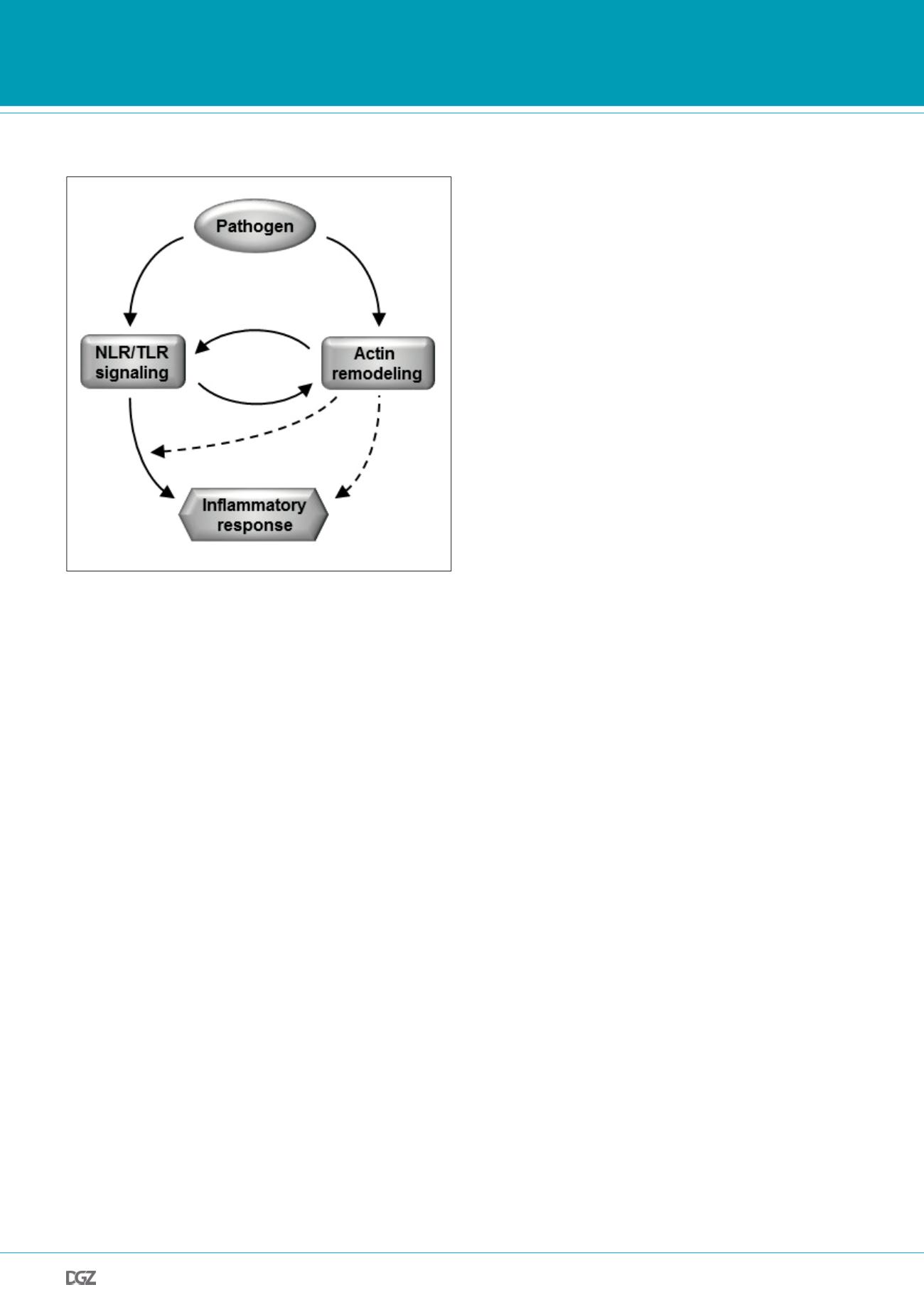
Figure 2:
Interplay of NLR/TLR signaling and actin remodeling in pathogen
induced inflammatory responses. During infection, innate immune respon-
ses are triggered upon recognition of PAMPs by PRRs like NLR or TLR. In
parallel, pathogens provoke reorganization of the host actin cytoskeleton,
e.g. to enable bacterial uptake. Besides, PRRs directly sense changes in
actin remodeling and integrate pathogen induced actin perturbations into
innate immune responses.


