16
Cell News 1/2015
Research news
Cellular force generation in focal adhesion
maturation and extracellular matrix remodeling
Jessica Morgner
1
and Sara A. Wickström
1, 2
1
Paul Gerson Unna Group ‘Skin Homeostasis and Ageing’, Max Planck Institute for Biology of Ageing,
Joseph-Stelzmann Strasse 9b, 50931 Cologne, Germany
2
CECAD Cologne Excellent in Aging Research, Joseph-Stelzmann Strasse 26, 50931 Cologne, Germany
Abstract
The extracellular matrix (ECM) functions as a structural scaffold
for tissues, but it also drives intracellular signaling by interac-
ting with specific receptors and by regulating the bioavailability
of growth factors. This unique combination of functions ma-
kes the ECM an important regulator of organ development and
maintenance. The deposition and remodeling of the ECM into
a precise configuration is a cell-dependent process that requi-
res integrin adhesion receptors as well as generation of cellular
forces. Integrin-linked kinase (ILK) is an essential adaptor prote-
in that binds to
β
1- and
β
3-integrin cytoplasmic tails and links
them to the actin cytoskeleton. Our work has uncovered func-
tions of ILK in cellular force transduction and ECM remodeling
and the role of these processes in cell fate regulation.
Interactions between cells with their neighbors and the envi-
ronment not only provide tissues their shape and proper ar-
chitecture, but also regulate fate decisions of individual cells.
Cell-matrix interactions have the potential to propagate signals
that regulate proliferation, differentiation, and migration, which
ensure coordinated cell behaviors during development and tis-
sue homeostasis (Wickström et al., 2011). Cells further actively
remodel the extracellular matrix (ECM) thereby engaging in
dynamic crosstalk with their environment (Daley and Yamada,
2013).
The ECM and integrins
The ECM is a complex non-cellular network composed mainly
of fibrous proteins and proteoglycans that determine the bio-
chemical and mechanical properties of the tissue. It serves as a
physical scaffold, but also as a platform for intercellular com-
munication and as a reservoir for growth factors. Therefore the
composition and organization of the ECM needs to be tightly
controlled (Frantz et al., 2010; Watt and Fujiwara, 2011; Gat-
tazzo et al., 2014).
Integrins are the major ECM receptors in all metazoans. Accor-
dingly, their main task is to facilitate the adhesion of cells to the
ECM. In addition, intracellular coupling of integrins to the actin
and intermediate filament cytoskeletons allows generation of
traction forces and regulation of cell shape and mechanics. Fi-
nally, integrins are able to assemble large intracellular signaling
platforms termed focal adhesions (FAs) that activate signaling
cascades. These unique features make integrins essential for a
large number of cellular processes (Legate et al., 2009; Wick-
ström et al., 2011).
Integrins comprise of 18
α
and 8
β
subunits that assemble non-
covalently into 24 distinct heterodimers (Hynes, 2002). The spe-
cific subunit combination determines their binding affinity and
ligand specificity. A hallmark of integrin receptors is their ability
to mediate bi-directional signaling. “Inside-out” signaling regu-
lates the ligand binding properties of integrins and is induced by
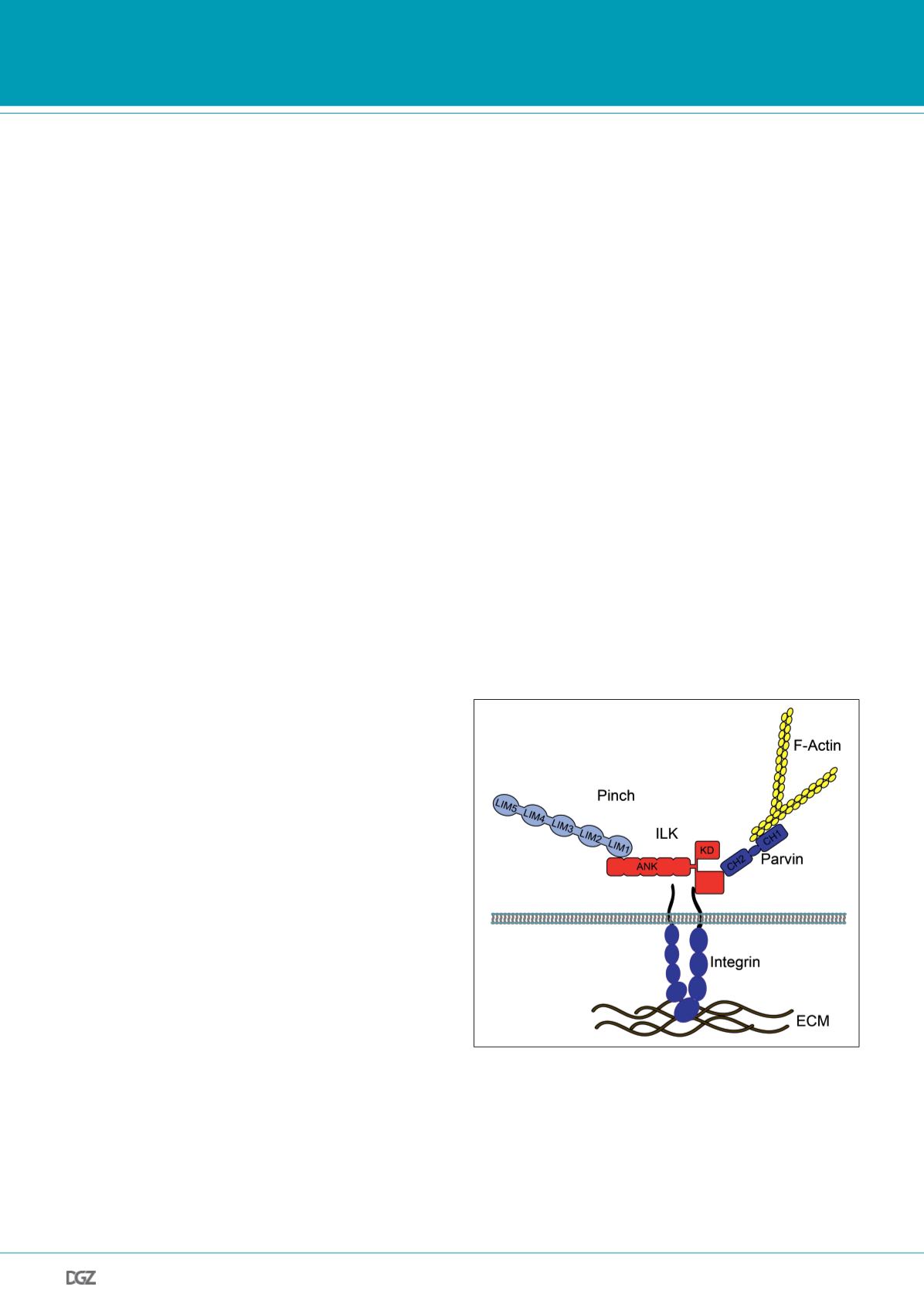
Figure 1: Integrin-linked kinase and its main binding partners
Integrin-linked kinase (ILK) is composed of two domains that are connec-
ted by a short linker: the N-terminal ankyrin repeat domain (ARD) and
a C-terminal kinase domain (KD). The ARD binds to the LIM1 domain of
particularly interesting Cys-His-rich protein (PINCH). The KD of ILK is a
protein-protein interaction domain that binds among others to the CH2
domain of parvins and the cytoplasmic tails of
β
integrins. Parvins are
capable of binding F-actin, thereby facilitating a direct link between inte-
grins and the actin cytoskeleton. It is not clear, however, whether ILK binds
β
integrins directly in vivo and whether parvins can bind actin while bound
to ILK (modified from Ghatak et al., 2013).


