Cell News 2/2014
19
ther example of substrate recognition is achieved by ¨phospho-
priming” for glycogen synthase kinase
3β
(GSK3
β
). This event is
crucial before ¨priming¨ or phosphorylation of its canonical sub-
strate. A phosphate group of a “primed” substrate associates to
a basic region of the GSK3
β
kinase domain. Then, GSK3
β
kinase
triggers downstream signaling pathways of the insulin receptor
tyrosine kinase (Fiol et al., 1990).
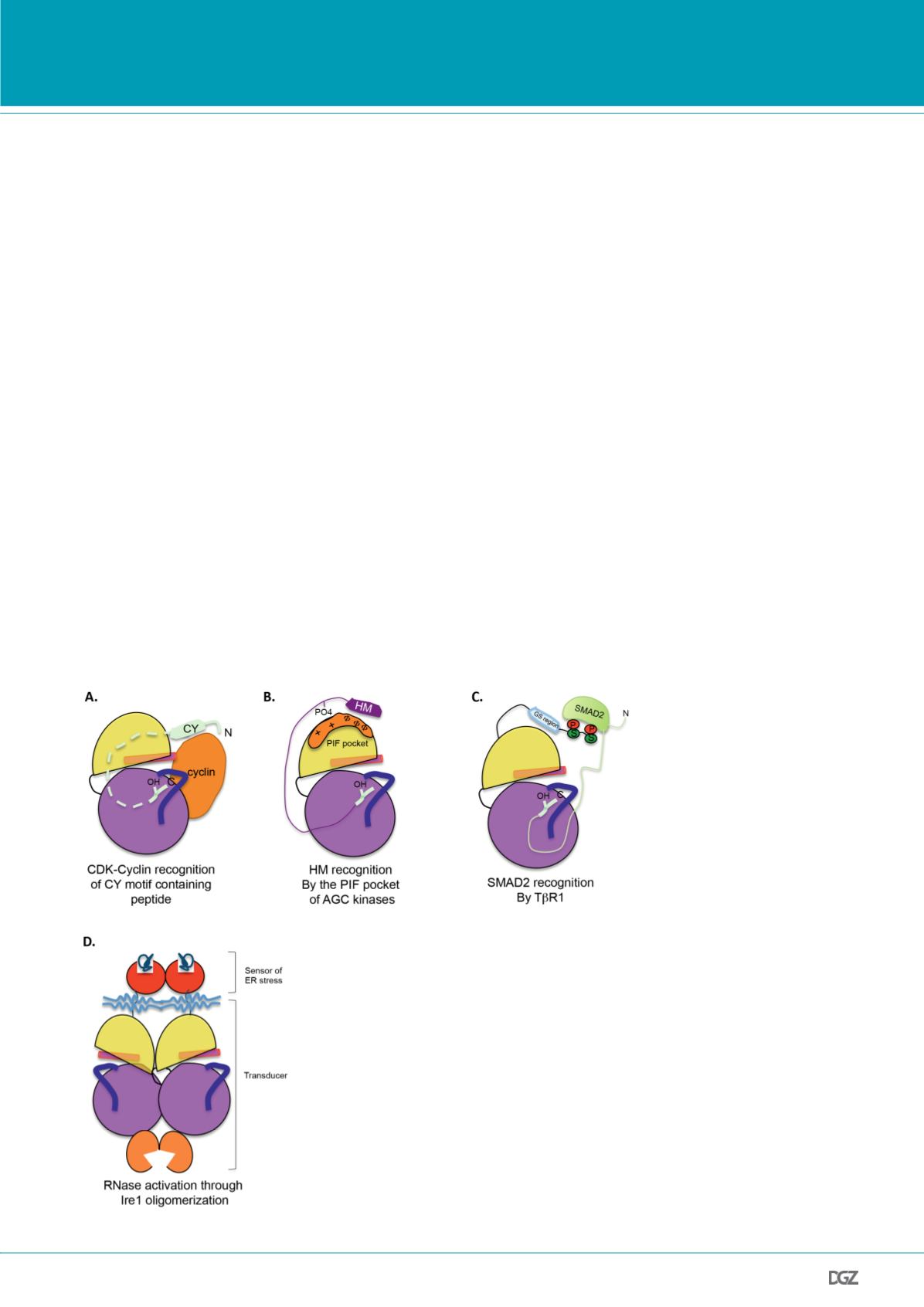
Transforming growth factor-beta (TGF-
β
) family members of the
TKL protein kinases group regulate a spectrum of cellular res-
ponses that range from control of early cell-fate decisions to
the induction of cancer (Siegel and Massage, 2003). The TGF-
β
family includes activins, nodals, bone morphogenetic proteins
(BMPs) and others. Downstream signaling of TGF
β
cell surface
protein kinases is achieved through the Smads, which are the di-
rect receptor substrates as well as non-Smad substrates (Reguly
and Wrana, 2003; Mu et al., 2012). Following phosphorylation,
the substrates accumulate in the nucleus to regulate transcrip-
tion through interactions with DNA binding proteins. The TGF
β
receptor family is composed of related type 1 (T
β
R1) and type
2 (
Tβ
R2) classes. Both co-receptors have an N-terminal extra-
cellular domain, which binds the ligand, a transmembrane regi-
on, and a C-terminal Ser/Thr kinase domain. Interestingly, T
β
R1
contains a GS region between the transmembrane and the ca-
talytic domain. Upon ligand binding to the TGF
β
receptor, T
β
R1
is activated by T
β
R2 through trans-phosphorylation of the GS
region (Vivien and Wrana, 1995). The full activation of T
β
R1 will
promote the recruitement of a Smad2 containing complex. This
event will then facilitate their nuclear accumulation and couples
them to the transcriptional machinery (Fig. 3A).
Ire1 is considered to be involved in one of the evolutionarily con-
served pathways of the unfolded protein response (UPR), with
bifunctional kinase-ribonuclease enzyme activities. Ire1 con-
tains an N-terminal endoplasmic reticulum (ER) lumenal domain
that directly senses unfolded proteins, a transmembrane region,
a kinase domain and a C-terminal KEN domain. Activation of
the ribonuclease function in Ire1 is achieved through binding
misfolded protein, which causes dimerization of the ER lumenal
domains and trans-autophosphorylation of the A-loop. Here, the
phosphorylated A-loop first permits ATP binding and then the
formation of a back-to-back Ire1 homodimer complex, which in
turn promotes the activation of the KEN domain with its ribo-
nuclease catalytic function (Fig. 3D) (Ken et al., 2008). The ac-
tivation segment of Ire1 regulates the ER lumenal ribonuclease
activity of its KEN domain.
Polo-like kinases (Plk) are a family of serine-threonine protein
kinases that are highly conserved from yeast to humans. They
play a variety of roles in the cell cycle progression. In mammals,
Plks1-5 have subsumed specialized functions. Plk1, which is re-
garded as the canonical Plk, functions at many sites and steps in
Figure 3.
Examples of high order structure of protein kinases with substrate targeting me-
chanisms based on domain-domain interactions. A. Bipartite substrate recognition sequences
with CY motif and peptide substrate phosphoacceptor site for cyclin-dependent protein
kinases. CDKs recognize a motif in their substrates through their regulatory cyclin dependent
partners. B. The AGC kinases target their substrates through a hydophobic motif (HM) in
the N-lobe, which promote the association of the HM to the PIF binding-pocket. C. SMAD2
substrate associates to TbR1 through the phosphoserines of the GS region. D. Oligomerization
activates Ire1, a bifunctional enzyme. The bifunctional kinase Ire1 contains a cytoplasmic
kinase domain connected to a ribonuclease (RNase) domain. Dimerization of lumenal domains
through association to misfolded protein causes the upregulation of ribonuclease activity by
the activation segment.


