Cell News 2/2014
11
Research news
Optical control of neuronal signaling proteins:
Activation of glutamate receptors with photoswitchable ligands
Andreas Reiner
Department of Molecular and Cell Biology, University of California, Berkeley
Abstract
Light offers unique advantages for studying and controlling
cellular processes. This perspective summarizes the develop-
ment and use of synthetic photoswitchable ligands for the op-
tical control of glutamate receptors.
Light is well suited to visualize and manipulate biological sys-
tems: It is orthogonal to most biological processes and there-
fore fairly non-invasive; at the same time it provides suffici-
ent spatial and temporal resolution to resolve most cellular
processes. Not surprisingly, the invention of light microscopy
marks the dawn of cell biology and advances in fluorescence
microscopy continue to widen our experimental abilities to the
present day. The discovery of fluorescent proteins provided us
with genetically encoded fluorophores, which are a particu-
larly valuable addition. We can control their expression and
use them to visualize cells or cellular compartments with high
specificity. Furthermore, we can use fluorescent proteins to tag
specific proteins in living cells, or to build sensors that report
on cellular signaling events in real time. In the past decade,
light has also become an increasingly valuable tool in another
way. Next to its use in imaging-based approaches, light is now
used for manipulating biological processes, e.g. by using light
sensitive proteins or chemical approaches to optically control
living cells.
Optogenetic tools for the control of cellular activity
Next to observing cellular processes it has been a long-standing
aspiration to manipulate cells with high specificity and spatio-
temporal resolution. This can be achieved by using light sensi-
tive (bio)molecules, e.g. by expressing light sensitive proteins in
specific cells or by deploying light sensitive ligands to specific
targets. A prominent example of these ‘optogenetic’ approa-
ches (Miesenböck, 2009; Miesenböck, 2011; Fenno et al., 2011)
is the control of individual neurons by expressing light-gated
ion channels, such as channelrhodopsin (Nagel et al., 2003).
Channelrhodopsin is a blue-light activated opsin isolated from
algae, which allows to robustly depolarize neurons and to trig-
ger action potentials (Boyden et al., 2005; Li et al., 2005). A key
advantage of such optogenetic tools (next to the advantages
inherent to optical techniques) is that their expression can be
genetically targeted to specific cell-types, which means that
specific cell populations can be manipulated in complex envi-
ronments (Fig. 1A). This is now widely used to study the role of
particular neurons in circuit function or in specific brain areas
of intact living animals (Tye, Deisseroth, 2012; Reiner, Isacoff,
2013). Next to channelrhodopsin, a number of other microbial
opsins is now employed to control neuronal excitability with
high precision (Zhang et al., 2011). For example, light-driven
transporters (pumps) can be used to hyperpolarize and silence
neurons.
Controlling the cellular membrane potential and thereby neu-
ronal excitability is an important step, in particular for addres-
sing neuronal circuit function. However, in many other cases it
is desirable to go beyond controlling the membrane potential
and to control specific signaling processes or even native sig-
naling proteins (Fig. 1B). Several strategies are used in this ra-
pidly expanding field. They either rely on naturally occurring
light sensitive signaling proteins (like light sensitive GPCRs,
adenylyl cyclases, etc.) (Zemelman et al., 2002; Li et al., 2005;
Schröder-Lang et al., 2007), or on protein domains that un-
dergo conformational changes upon illumination (e.g. flavin-
containing BLUF and LOV domains) (Möglich, Moffat, 2010).
The latter can be utilized as optical actuators by fusing them
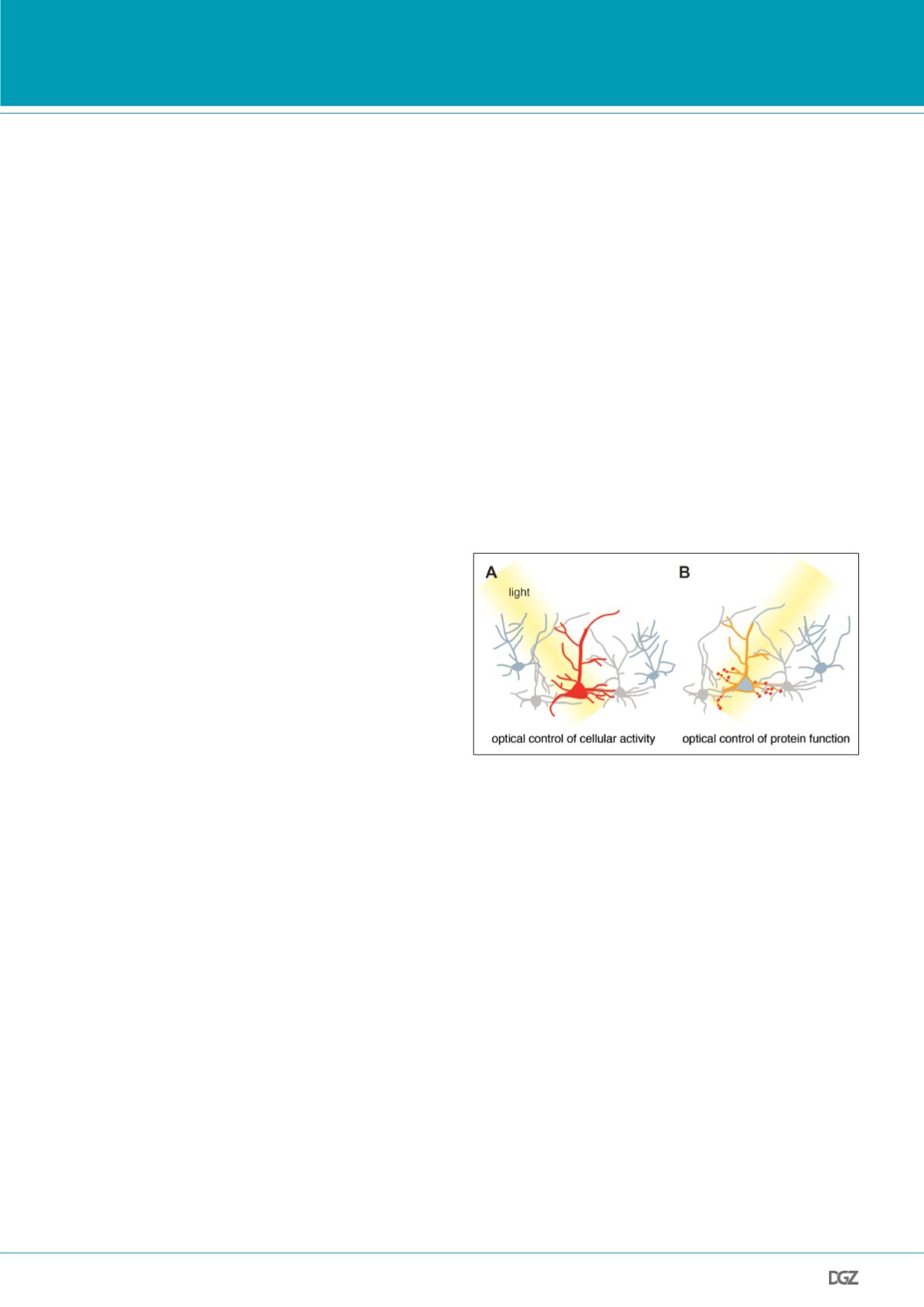
Figure 1. Optogenetic manipulation of specific cells and proteins.
(A) Specific cells (red) can be optically manipulated by expressing light
sensitive proteins. For instance, microbial opsins, which act as light sen-
sitive ion channels or transporters, can be expressed in neurons to control
the membrane resting potential and thereby neuronal firing. This approach
is now widely used to probe the role of specific neurons in complex cir-
cuits. (B) In other cases it might be desirable to use optogenetic tools for
controlling the function of specific proteins (red) and signaling processes
endogenous to the cell. For example, synthetic photoswitchable tethered
ligands can be used to probe the physiological role of specific cell surface
receptors involved in synaptic signaling. In both cases, genetic approaches,
such as driver lines or cell-type specific promoters, are used to target the
manipulation to a selected subset of cells.


