Cell News 2/2014
6
Life with nuclear actin filaments
Matthias Plessner, Pilar Chinchilla, Christian Baarlink
and Robert Grosse
Introduction
As part of the cytoskeleton, actin is one of the most abundant
cellular protein and hence involved in many different processes,
for instance maintaining cell shape or generating contractile
force in the context of cell motility and cytokinesis [1]. These
functions are related to dynamic changes in actin structures.
Generally, actin can be found in two different states. It can ap-
pear as either a monomeric, globular protein called G-actin or
as part of a polymeric, elongated microfilament termed F-actin
[2].
The assembly of G- actin subunits into trimers is termed actin
nucleation. This process constitutes the initial step in the for-
mation of stable microfilaments. Formins, Arp2/3 complex and
Spire are the three major classes of actin nucleators. Arp2/3
and Spire both bind to pointed ends of actin filaments. While
the Arp2/3 complex organizes actin filaments into branched
networks, Spire has the ability to assemble linear filaments [3].
Formins polymerize linear filaments as well, although they bind
to barbed instead of pointed ends [4]. As a key feature, all for-
mins contain two formin homology (FH) domains, termed FH1
and FH2 domains. The FH2 domains form a circular head-to-tail
homo-dimer thereby stabilizing actin dimers and adding them
to the barbed end in a stair-stepping process, while binding of
actin by the FH1 domain increases the local G-actin concentra-
tion to accelerate actin polymerization [5].
Among the different classes of formins, diaphanous-related for-
mins (DRFs) are best characterized [6]. DRFs show a modular do-
main organization in which the regulatory segment is composed
of a GTPase binding domain (GBD) and a diaphanous-inhibitory-
domain (DID), both of which are involved in the autoinhibitory
regulation of DRFs. The FH1 and FH2 domains are located at
the C-terminus together with the diaphanous-autoregulatory-
domain (DAD). In the dormant state, DAD binds DID to achieve
autoinhibition, which further blocks the polymerization of actin
filaments by the FH2 domain. Additive binding of a Rho GTPase
can release autoinhibition by sterically influencing the DID-DAD
interaction [7, 8]. Nevertheless, the DID-DAD autoinhibitory
module is influenced by various cellular signaling processes in-
cluding serine/threonine kinases [8].
Actin filaments display structural polarity because G-actin mo-
nomers within a filament are oriented in the same direction.
Based on their appearance in electron microscopy, the terminal
part is referred to as either barbed or pointed end [9]. Many
proteins affect actin dynamics and can in fact influence the rate
of actin assembly as well as secondary structures of F-actin.
Examples of secondary structures are actin bundles, networks of
branched actin filaments and the actomyosin ring responsible
for cytokinesis [2].
Research news
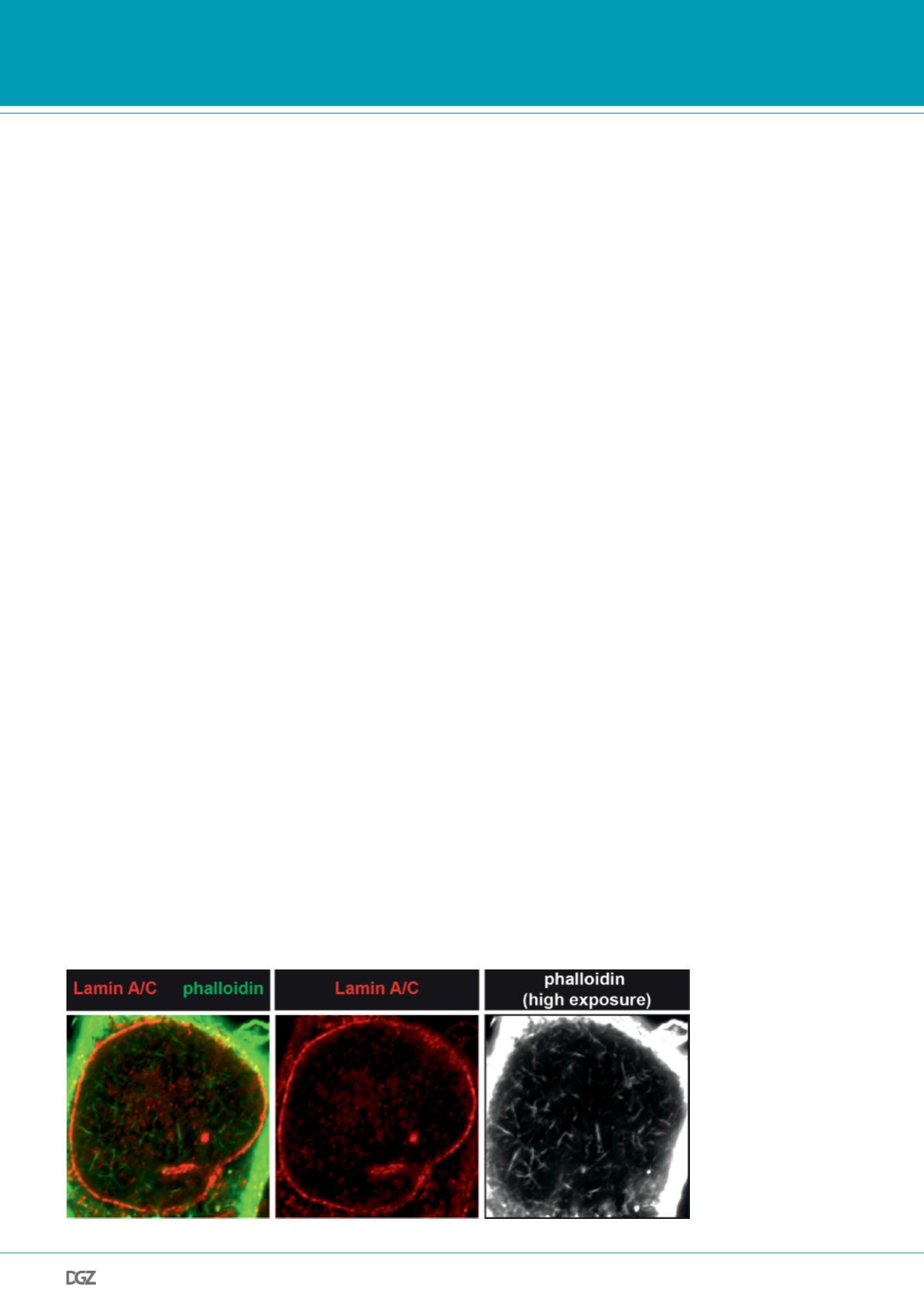
Figure 1. Visualization of
nuclear actin filaments by
phalloidin staining.
M2 melanoma cells were
kept in serum-free medium
before stimulation with 20%
FCS for 90 s and immediate
glutaraldehyde fixation. Cells
were stained for lamin A/C
(red) and actin filaments using
phalloidin (green; white in
right panel).


