Cell News 2/2014
7
Research news
Nuclear actin polymerizes
With regard to the nucleus, the structure and function of actin
is still a matter of debate [10, 11]. This is to some extent caused
by difficulties in visualizing nuclear actin. Therefore, a paradigm
has prevailed for decades stating that nuclear actin only exists
in its monomeric form or hypothetically as very short filaments
[12, 13]. However, our view on nuclear actin structures is rapidly
expanding. We recently described the existence of a formin-de-
pendent, dynamic F-actin network in the nucleus, which forms
rapidly upon stimulation with serum [14]. To visualize and study
nuclear actin filaments in living cells, we have targeted the ac-
tin probe LifeAct to the nucleus, thereby avoiding massive signal
interference from cytoplasmic actin labeling [15]. Importantly,
serum-stimulated nuclear F-actin assembly can also be detec-
ted without ectopic protein expression by the bona fide F-actin
marker phalloidin, evidencing the existence of native nuclear
actin filaments in somatic cell nuclei (fig. 1) [14].
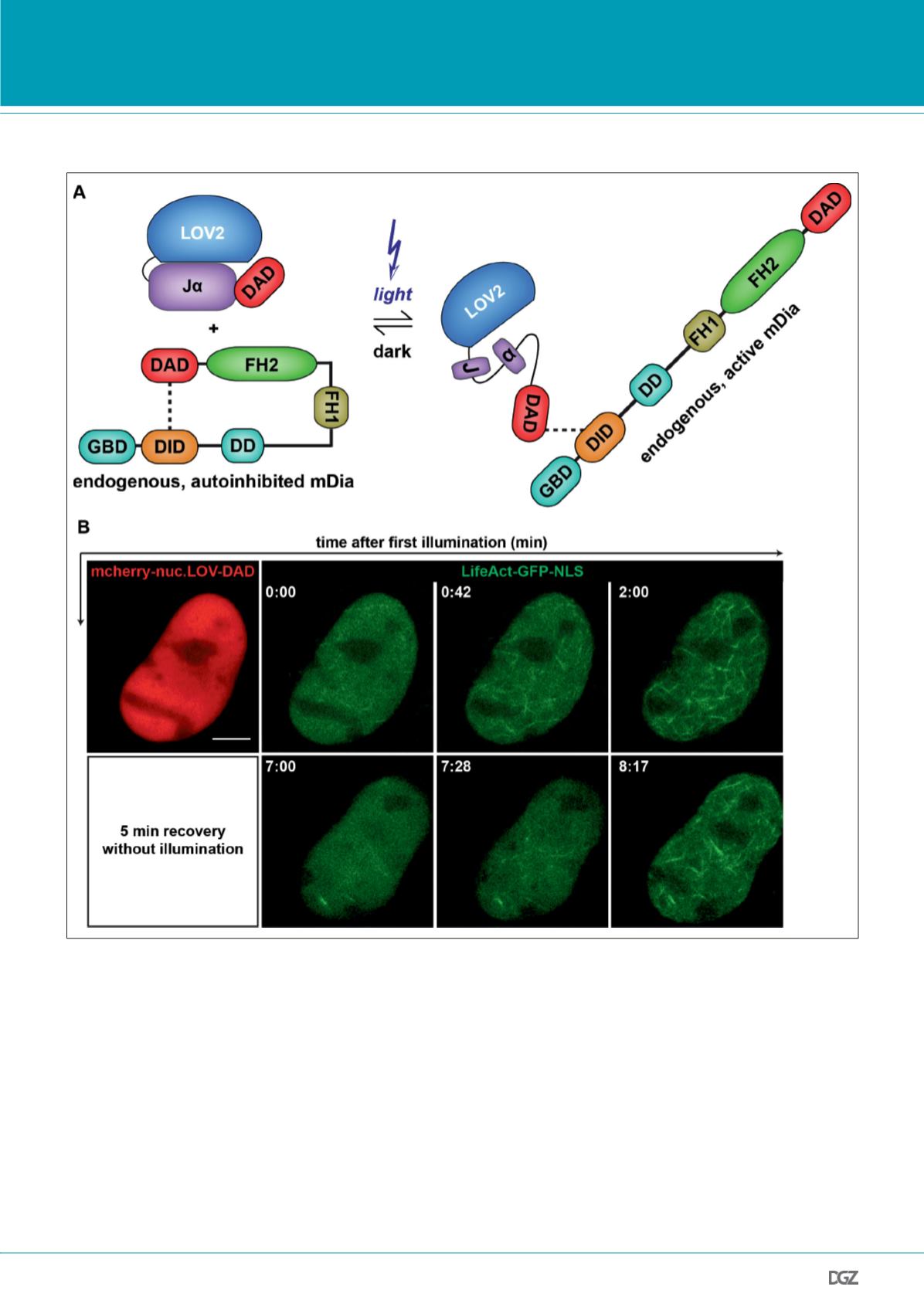
Figure 2. Actin network assembly upon activation of nuclear mDia by a photoactivatable DAD (nuc.LOV-DAD).
(A) Cartoon illustrating the light-induced ability of LOV-DAD to activate endogenous mDia. The LOV2 domain and the adjacent c-terminal J
α
-helix are
shown. Illumination with blue light leads to unfolding of the J
α
-helix and subsequently “uncages” the fused DAD domain.
(B) Optogenetic activation of endogenous nuclear mDia induces nuclear actin filament formation. NIH3T3 cell expressing mCherry-nuc.LOV-DAD was
repeatedly irradiated with 488 nm to simultaneously activate nuc.LOV-DAD and to visualize LifeAct-GFP-NLS. Scale bar represents 5 µm.


