Cell News 2/2014
8
Research news
The Serum Response Factor (SRF) is under control of
nuclear actin polymerization by formins
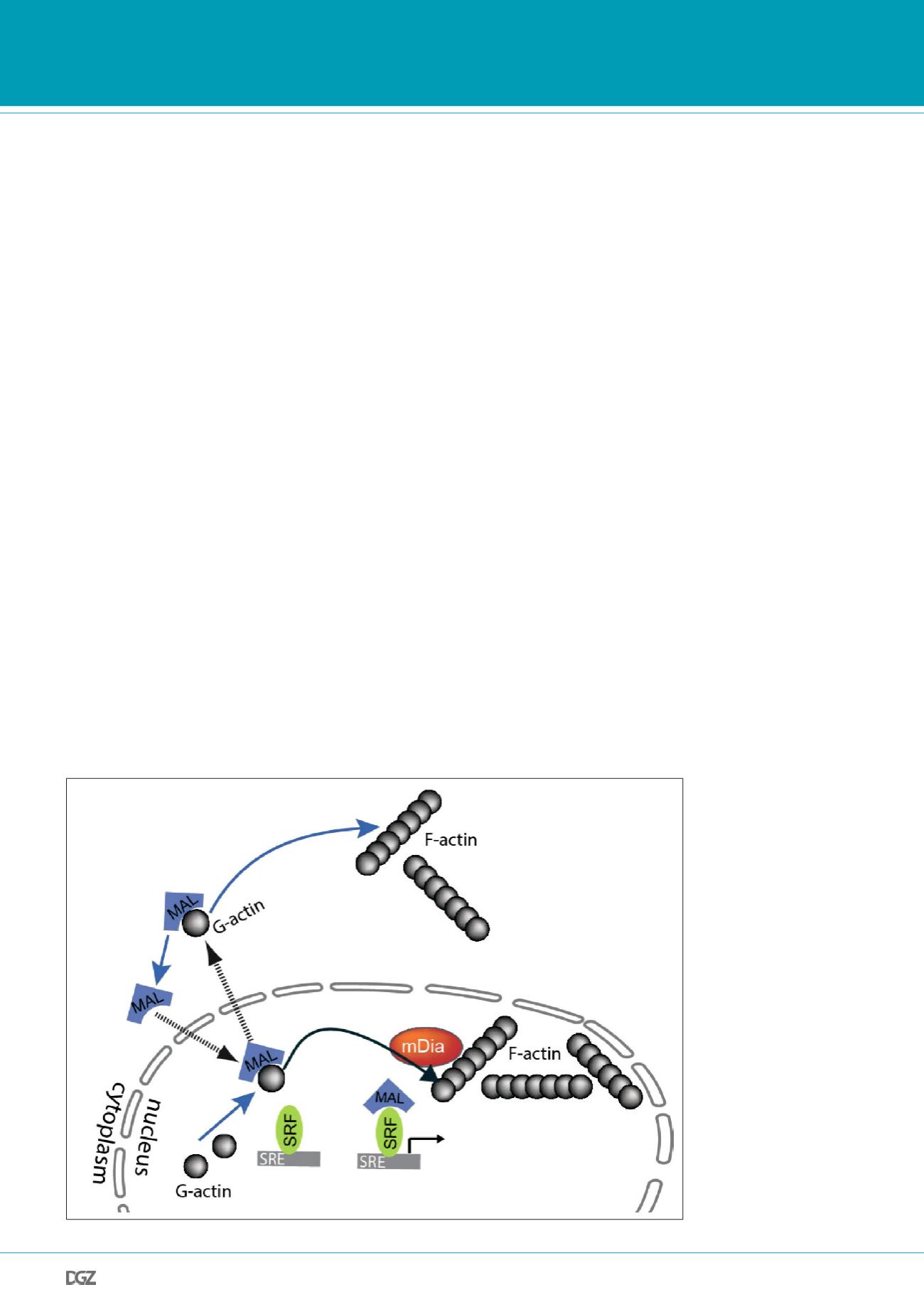
As part of the serum response, nuclear F-actin dynamics are
functionally linked to megakaryocytic acute leukemia (MAL)
protein (also termed myocardin-related transcription factor A;
MRTF-A), which acts as a transcriptional coactivator of serum
response factor (SRF) [16]. MAL is continuously and rapidly shut-
tling between the cytoplasmic and nuclear compartment and
hence its activity is controlled by its localization, which in turn
is regulated by compartmentalized actin polymerization [17;
18]. When loaded with G-actin, MAL is predestined for nuclear
export to the cytoplasm while in the cytoplasm G-actin-binding
can interfere with access to the NLS (nuclear localization signal)
of MAL. Upon release from G-actin, MAL cannot be exported
from the nucleus and thus promotes SRF-mediated transcrip-
tion [18]. In order to switch off MAL activity, MAL has to bind
nuclear G-actin and re-translocate to the cytoplasm [19]. We
could demonstrate a mechanism in which formin-dependent
polymerization of actin in the nucleus appears to be a critical
step in mammalian cells, which is required to prevent nuclear
export of MAL to control SRF activity in response to extracellu-
lar signals [14, 15].
To study the functional role of endogenous nuclear formins, we
made use of the power of optogenetics by generating a light-
switchable tool for DRF activation. By fusing the LOV (light,
oxygen, or voltage) J
α
-domain of Avena sativa phototropin-1
[20-22] to the DAD region of mDia2, we could spatiotemporally
unleash endogenous formin autoinhibition by light-induced un-
caging of the DAD (fig. 2A). Remarkably, this approach of a pho-
toactivatable DAD efficiently and reversibly stimulated the for-
mation of long and unbranched nuclear actin filaments (fig. 2B).
As a consequence, MAL rapidly relocated from the cytoplasm to
the nuclear compartment, demonstrating the importance and
efficiency of nuclear actin polymerization for MAL regulation
(fig. 3) [14].
Besides regulating MAL/SRF activity, there are likely additional
functions for F-actin inside somatic cell nuclei. As monomeric
actin is involved in many gene regulatory processes like chro-
matin remodeling and mRNA processing, it will now be exciting
to determine the possible functions of nuclear actin assembly
and F-actin structures for these processes as well as cell motili-
ty, adhesion and differentiation.
References
1. Rottner, K. and T.E. Stradal, Actin dynamics and turnover in cell motility. Curr Opin Cell
Biol, 2011. 23(5): p. 569-78.
2. Pollard, T.D. and J.A. Cooper, Actin, a central player in cell shape and movement. Sci-
ence, 2009. 326(5957): p. 1208-12.
3. Kerkhoff, E., Cellular functions of the Spir actin-nucleation factors. Trends Cell Biol,
2006. 16(9): p. 477-83.
4. Faix, J. and R. Grosse, Staying in shape with formins. Dev Cell, 2006. 10(6): p. 693-706.
5. Campellone, K.G. and M.D. Welch, A nucleator arms race: cellular control of actin as-
sembly. Nat Rev Mol Cell Biol, 2010. 11(4): p. 237-51.
6. Bogdan, S., J. Schultz, and J. Grosshans, Formin' cellular structures: Physiological roles
of Diaphanous (Dia) in actin dynamics. Commun Integr Biol, 2013. 6(6): p. e27634.
7. Lammers, M., et al., The regulation of mDia1 by autoinhibition and its release by
Rho*GTP. EMBO J, 2005. 24(23): p. 4176-87.
Figure 3. Cartoon illustrating
the interplay between actin
dynamics and nuclear MAL/
SRF activity.
MAL export is regulated by
nuclear actin dynamics and
requires MAL-actin complex
formation. Nuclear actin
polymerization is required for
G-actin dissociation from MAL
(MRTF-A) thereby blocking
MAL export for subsequent
MAL/SRF-mediated transcrip-
tional activity.


