Cell News 2/2014
12
to the signaling protein of interest and this approach has been
applied to control gene expression, protein localization or en-
zymatic activity. A third successfully used strategy is to rely
on synthetic photoswitches to control native, genetically en-
gineered signaling proteins, such as receptors located at the
cell surface.
Optochemical approaches to control specific receptors
at the cell surface
Many cellular signaling events result from communication
between cells, which are often triggered by the binding of
ligands to cell surface receptors. For example, the ligand-in-
duced activation of G protein-coupled receptors (GPCRs) or of
ligand-gated ion channels leads to second messenger signaling
and ion fluxes across the cell membrane, respectively. Both,
for triggering such signaling processes and for studying the
physiological consequences of receptor activation, it would
be ideal to control ligand binding with light. Indeed, such si-
gnaling complexes can be directly rendered light sensitive by
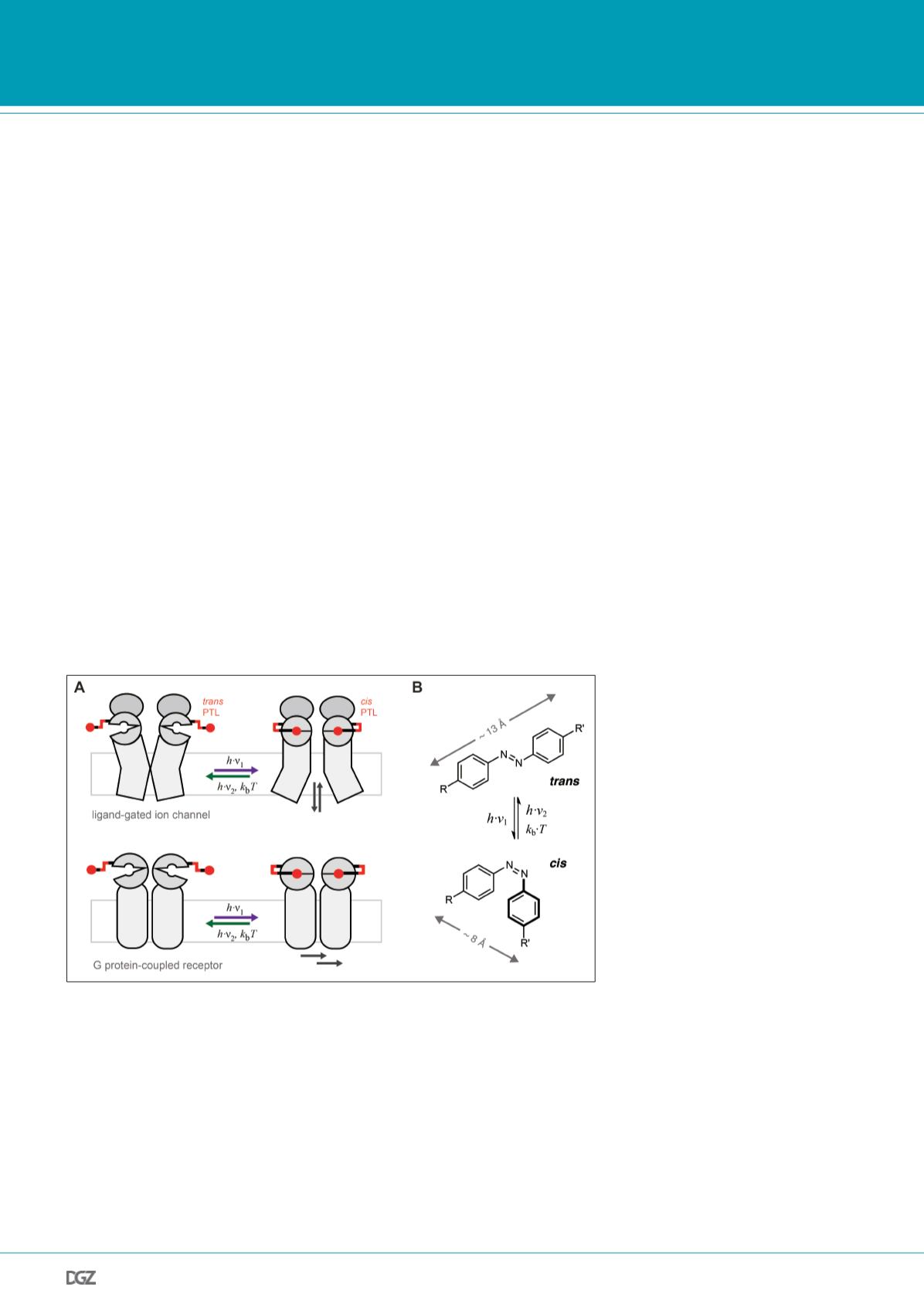
employing photoswitchable tethered ligands (PTLs) that can be
switched between two conformations, one that allows ligand
binding and receptor activation, and one that is inert and does
not activate the receptor (Fig. 2A) (Volgraf et al., 2006; Goros-
tiza, Isacoff, 2008). These PTLs are attached to the receptor of
interest via a cysteine anchor close to the ligand binding site,
which ensures high molecular specificity and limits the mani-
pulation to cells expressing the mutant receptor subunits.
While light-sensitive proteins rely on photoswitchable cofac-
tors such as retinal, synthetic photoswitches are often based
on azobenzene compounds (Fig. 2B). Azobenzenes can be pho-
toisomerized between a trans and a cis configuration using
light of different wavelengths, and are well tolerated by biolo-
gical systems. The cis/trans-photoisomerization of azobenzene
is fully reversible and provides a relatively large conformational
change. To construct a PTL, one side of the azobenzene core
is modified with a cysteine-reactive group for attachment to
the receptor, whereas the other side is functionalized with a
ligand, e.g. an agonist that leads to receptor activation when
bound. This ligand headgroup can resemble the endogenous
ligand or it can be derived from synthetic ligands, such as
agonists, antagonists or allosteric modulators, that exist for
many signaling complexes. The switching properties of the PTL,
namely the wavelength dependence of the photoisomerization
and the thermal cis-to-trans relaxation (off behavior) can be
rationally tuned by chemical modifications to the azobenze-
ne core (Kienzler, Reiner et al., 2013). Overall the use of PTLs
constitutes a modular and versatile optogenetic approach that
can be used to photo-activate or photo-inhibit native signa-
ling complexes with light. In the following, I summarize work
illustrating how PTLs can be used to photo-activate glutamate
receptors, a class of receptors that plays a key role in neuronal
signaling.
Optical control of ionotropic glutamate receptors
Glutamate is the major excitatory neurotransmitter in the cen-
tral nervous systems, which mediates excitatory neurotrans-
mission, but also modulates synaptic strength and plasticity.
It acts through binding to ligand-gated
ion channels (ionotropic glutamate re-
ceptors, iGluRs) (Traynelis et al., 2010)
and GPCRs (metabotropic glutamate
receptors, mGluRs) (Niswender, Conn,
2010), which are localized in pre- and
postsynaptic regions, as well as glial
cells surrounding the synapse. iGluRs
are tetrameric ion channels formed
by four subunits, which form a central
pore and encompass four glutamate
binding sites. The presence of diffe-
rent family members with overlapping
functions and similar pharmacology
makes it often difficult to study their
exact role in neurotransmission. For
example, 18 different iGluRs have been
described, which are mainly grouped in
three subfamilies, AMPA, kainate and
NMDA receptor subtypes (Hollmann,
Heinemann, 1994). The functional di-
versity of iGluRs is further increased by
editing and splicing isoforms, as well as
by formation of heteromeric complexes
that incorporate different subunit types
(Hollmann, Heinemann, 1994; Traynelis et al., 2010). In addi-
tion, iGluRs operate on fast timescales, that is ligand-induced
gating occurs within milliseconds. Optical control of iGluRs is
therefore highly desirable, as it may allow to overcome some
of the limitations encountered with conventional approaches.
Figure 2. Photoactivation of cell surface receptors with photoswitchable tethered ligands (PTLs).
(A) A synthetic ligand (red) that can be switched between a binding incompetent and a binding compe-
tent conformation is attached to the receptor of interest (grey), a strategy that can be used to activate
ligand-gated ion channels (top) or G protein-coupled receptors (GPCRs, bottom). (B) Azobenzene serves
as photoswitch. The azobenzene group can be photoisomerized between an extended trans configura-
tion and a ‘bent’ cis configuration by illumination with light of different wavelengths. One side of the
azobenzene group is used to covalently attach the ligand to the receptor of interest (R), the other side
to install a functional ligand (R’).
Research news


