Cell News 2/2014
13
Research news
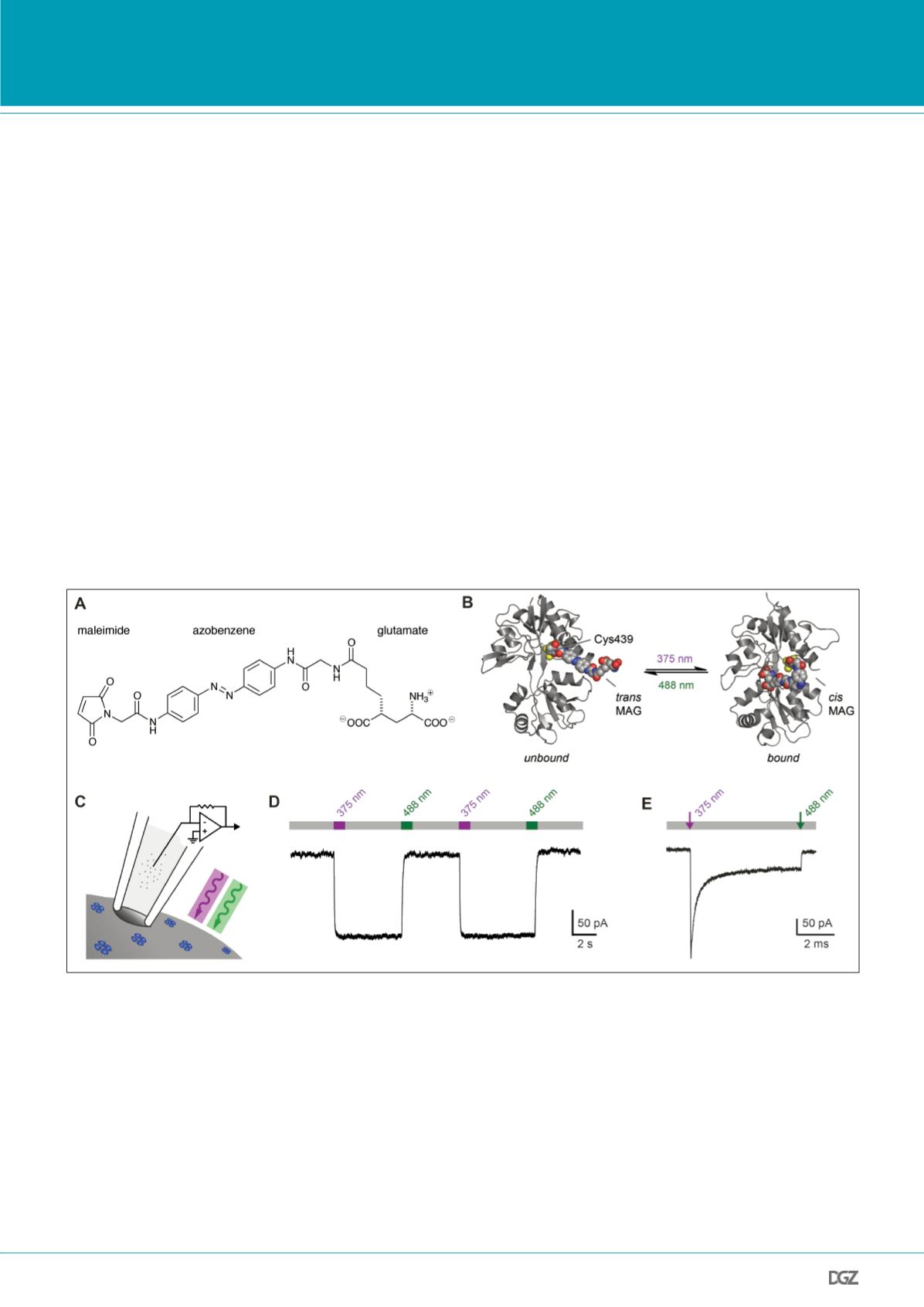
The use of PTLs allows to control specific iGluR subtypes with
high spatial and temporal precision. For instance, specific con-
trol of GluK2 (formerly named iGluR6), a member of the kainate
receptor family, can be achieved by expressing an engineered
GluK2 variant that carries a cysteine substitution in close pro-
ximity to the binding pocket (L439C) and covalently attaching
a MAG-type PTL to this residue (Fig. 3) (Volgraf et al., 2006;
Gorostiza et al., 2007; Reiner, Isacoff, 2014a). Light can then
be used to selectively activate and deactivate the PTL-labeled
GluK2 receptors, which results in an inward current, as seen in
patch-clamp recordings (Fig. 3D) or by Ca
2+
-imaging (Volgraf
et al., 2006).
Although a chemical photoswitch is employed, the receptor
substitution is still genetically encoded. As such, light-gated
glutamate receptors (LiGluRs) can be expressed in various cell
types, including neurons to control neuronal excitability (Szo-
bota et al., 2007), in astrocytes to control Ca
2+
-signaling (Li
et al., 2012), or in neuroendocrine cells to control exocytosis
(Izquierdo-Serra et al., 2013). A potassium selective version
of LiGluR has been engineered to silence neurons through hy-
perpolarization (Janovjak et al., 2010). The use of light-gated
iGluRs is not limited to cultured cells, but they can be also used
in vivo
: For instance, viral expression in retinal ganglion cells
allows to restore basic light sensitivity to retinas, which have
lost their photoreceptor cells due to degeneration (Caporale et
al., 2011). Another example is the use of light-gated iGluRs as
optogenetic tools to identify neuronal subpopulations and to
dissect their contributions to circuit function. Controlled ex-
pression in transgenic Gal4 driver-lines helped to clarify how
different types of neurons in the spinal cord control the swim-
ming of zebrafish larvae (Wyart et al., 2009). Light-gated iG-
luRs are also well-suited to distinguish pre- and postsynaptic
effects, which is often difficult to achieve with conventional
approaches. For example, the exclusive activation of postsyn-
aptic receptors allows studying retrograde effects on presyn-
aptic cells, as demonstrated at the fly neuromuscular junction
(Kauwe, Isacoff, 2013).
Figure 2. Optical control of ionotropic glutamate receptors (iGluRs).
(A) PTLs for controlling iGluRs, called MAGs, consist of a maleimide group for covalent labeling of cysteines, the azobenzene photoswitch, and a glutamate
derivative that acts as high efficacy ligand (the trans isomer is shown). This ligand is switched to the cis configuration with near-UV light (~375 nm) and
green light (~480 nm) can be used to switch it back to trans (Gorostiza et al., 2007). (B) The PTL is tethered to the ligand binding domain of GluK2 using
a cysteine substitution (L439C). In the trans configuration the ligand cannot reach the binding site, whereas it acts as high efficacy agonist in the cis
configuration. The model shows a single, MAG-labeled ligand binding domain. Adapted from Gorostiza, et al. 2007; Copyright (2007) National Academy
of Sciences, USA. (C) Engineered GluK2 receptors (blue) are expressed in the cells of interest and labeled with MAG. Photoswitching of these ligand-gated
ion channels can be verified in whole cell voltage-clamp recordings. (D) Photoactivation of MAG-labeled GluK2(439C) with 375 nm light leads to channel
opening, which results in an inward current. The process can be fully reversed by illumination with 488 nm light (two switching cycles are shown, the
grey bar denotes darkness). HEK cell voltage-clamp recording in the presence of the desensitization blocker concanavalin A (Con A), U
h
= -70 mV.
(E) Photoswitching with high time resolution. A short pulse at high light intensity (100 µs) is sufficient to trigger ligand binding and causes channel
opening in less than a millisecond. Activation is followed by ligand-induced desensitization on the millisecond timescale, resembling the activation by
glutamate (voltage-clamp recording in the absence of Con A). Reprinted from Reiner and Isacoff, 2014b.


