Cell News 2/2014
18
The generation of an intramolecular inhibitory element through
structured domains is a third type of regulation in protein ki-
nases. Several examples of kinase inhibitory states are shown
by X-ray crystallographic analysis. The inactive conformation of
the cyclin-dependent kinase CDK2 is achieved by the lateral dis-
placement of the N-lobe helix
α
C when lacking the binding to
cyclin and A-loop phosphorylation. The A-loop phosphorylation
releases the inhibition of the catalytic activity of the CDK2 kina-
se domain. Here, bipartite substrate recognition by CDKs occurs
through a C-terminal CY peptide motif, which recognizes their
regulatory substrate cyclin-binding partners (Fig. 3A).
Src tyrosine protein kinases contain in addition to an N-terminal
myristylation signal, an SH3 domain, an SH2 domain, a tyrosi-
ne catalytic domain and a C-terminal inhibitory linear sequence
with a tyrosine phospho-regulatory site. All the three compo-
nents of the Src family kinase, SH3 domain, SH2 domain and
the C-terminal inhibitory linear sequence inhibit in a concerted
action the catalytic activity of the kinase domain (Sicheri et al.,
1997; Xu et al., 1997). Autoinhibition of the catalytic activity
of the Src family kinase domain comes about by the intramole-
cular displacement of the SH2 domain with the phosphorylated
C-terminal tyrosine key residue and the SH3 domain with the
proline helical stretch ligand, respectively, on the back of the
kinase domain (Fig. 2C). This high order intramolecular struc-
ture achieves a non-productive kinase domain conformation by
lateral displacement of the N-lobe helix
α
C away from the C-
lobe A-loop. The activation of Src protein kinases is achieved by
phosphorylation of the A-loop and dissociation of the SH2 and
SH3 domains from the kinase domain by binding to high-affinity
the C-terminal tail of the key phospho-tyrosine residues and
proline-rich ligands, respectively (Fig. 2C) (Sicheri et al., 1997;
Xu et al., 1997).
Versatile Secondary Determinants for
Protein Kinase Substrate Recognition
A-loop activation of protein kinases is achieved through bin-
ding phospho-acceptor site. Protein kinases have the ability to
discriminate targeted Ser/Thr and Tyr phospho-acceptor sites of
their substrates. Furthermore, flanking sequences of the phos-
pho-acceptor sites are critical for the substrate recognition and
activation specificity, which is called canonical peptide substrate
recognition. Prediction programs based on Ser/Thr kinase domain
structures in complex with a peptide substrate can be used in
order to determine the minimal consensus motif of the protein
kinase substrate. Here, it is important to validate the predictive
interacting substrate through experimentation (Brinkworth et
al., 2003).
The AGC protein kinases including PKB/Akt target their subs-
trates through a regulatory element called hydrophobic motif
(HM) located at the C-terminal region to the AGC kinase domain
(Pearson et al., 1995). The hydrophobic motif possesses a phos-
pho-acceptor Ser or Thr residue, surrounded by large aromatic
phenylalanine or tyrosine residues with consensus motif Phe-X-
X-Phe-Ser/Thr-Phe/Tyr (Pearson et al., 1995) (Fig. 3B). The AGC
protein kinases contain a conserved region in their N-lobe called
PIF pocket (Fig. 3B). The PIF pocket associates to the complemen-
tary binding site for the hydrophobic motif in its substrate. Ano-
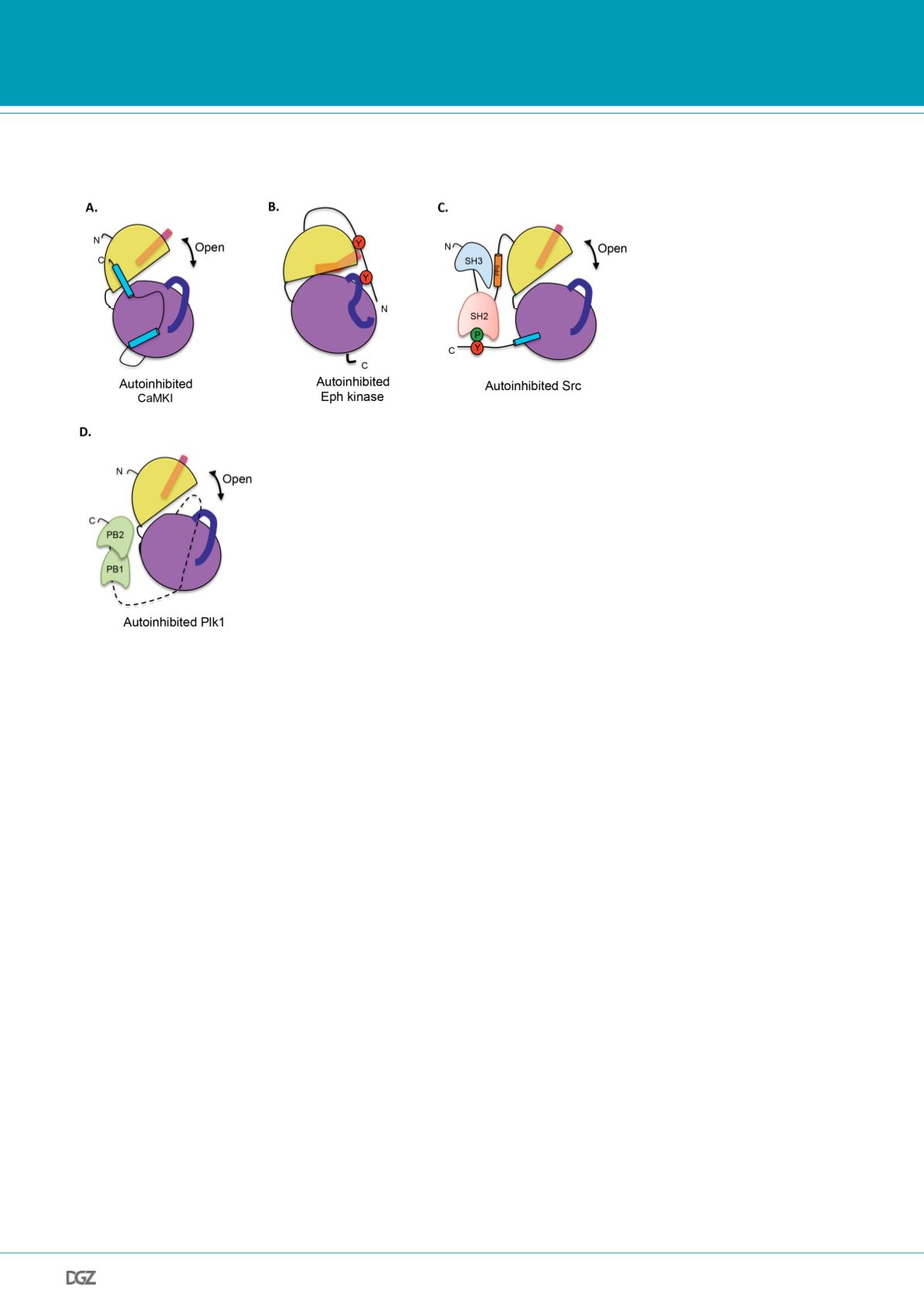
Figure 2.
Examples of catalytic activity inhibition for protein kinases. Representation of the kinase domain
with conserved structural elements are highlighted, depicting known mechanisms of protein kinase inhibiti-
on. A. CaMKI autoinhibited by a pseudosubstrate. The C-terminal tail of CaMKI binds to the N-lobe to make
an open, non productive conformation of the kinase domain. B. Autoinhibition of the catalytic activity of the
Eph tyrosine receptor by the juxtamembrane region. Unphosphorylated tyrosine residues of the juxtamemb-
rane region binds to the N-lobe of the kinase domain, promoting a displacement in helix aC and a disordered
A-loop. C. Autoinhibition of the Src family kinases by intramolecular interaction of the SH2 domain with the
phosphorylated C-terminal region and the SH3 domain with a polyproline (PPII) helical structure. The binding
of the intramolecular domains and motifs makes a stable non catalytic conformation and a displacement
of the helix aC. D. Autohinibition of Plk1 kinase by intramolecular association with PB1 and PB2 domains.
Plk1 is autoinhibited in the resting state, which can be activated through association of PB1 and PB2 with a
phosphopeptide ligand.


