Cell News 4/2014
22
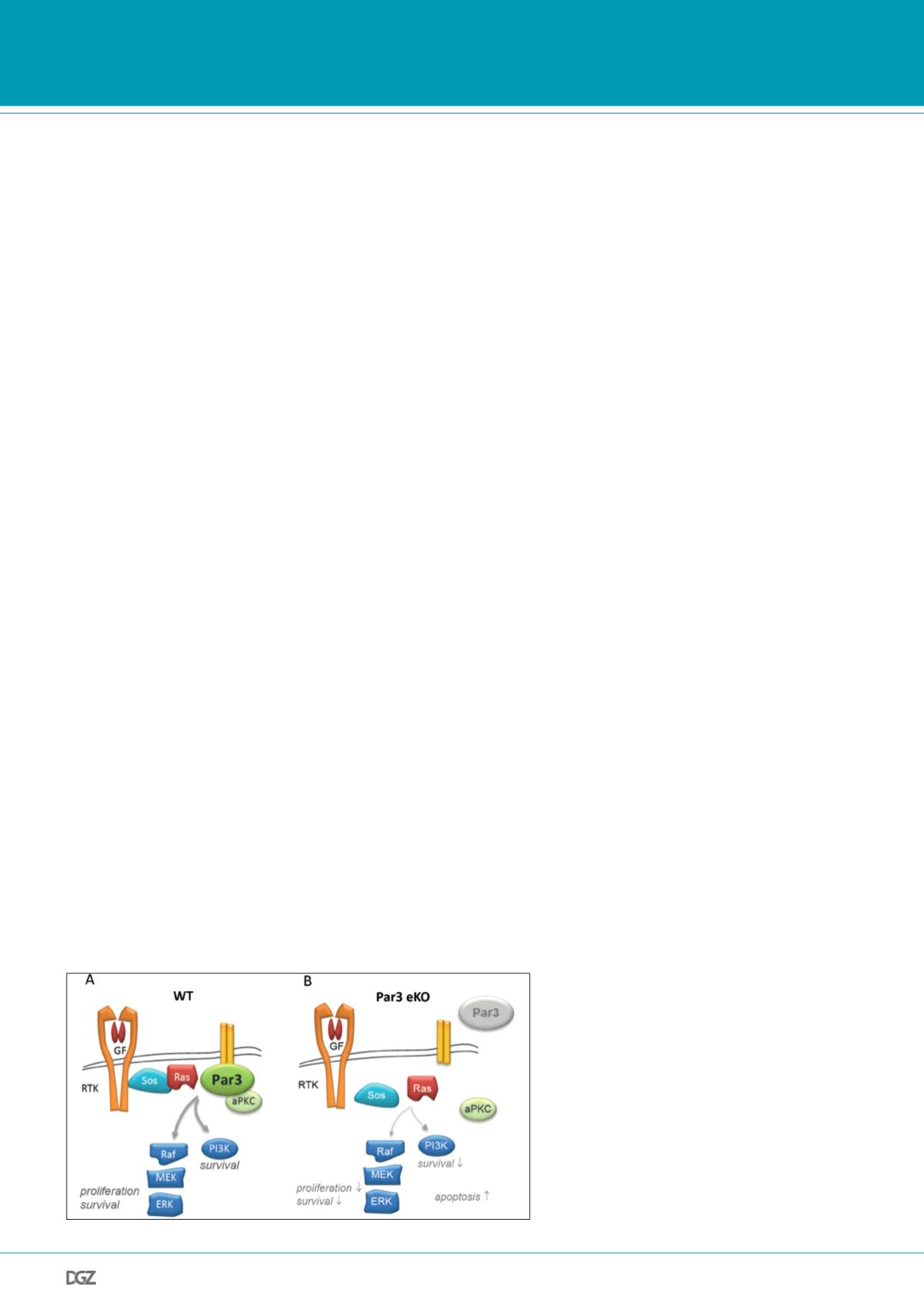
Figure 4: Model of tumor-promoting function of Par3
in contacting keratinocytes and skin papilloma.
A, In wild-type murine keratinocytes harboring firm inter-
cellular adhesions, Par3 localizes to cell-cell contacts and
serves to recruit aPKC to this site. Par3 moreover stabilizes
Ras signaling components incl. Sos, Ras, and phosphoryla-
ted ERK to cell-cell contacts, colocalizing there with ZO-1
(not shown here). This assembly of a signaling-competent
protein network is required for robust Ras-Raf-MEK-ERK
and PI3K/Akt activation resulting in proliferation and
survival signals. B, Loss of epidermal Par3 results in strong
reduction of cortical aPKC, Ras, Sos, and active ERK. aPKC
instead is enriched in the cytoplasm. As consequence,
proliferation and survival signals are strongly reduced in
contacting Par3 KO cells, and apoptosis via activation of
the intrinsic pathway (not shown) is increased. RTK, recep-
tor tyrosine kinase; GF, growth factor; WT, wild-type.
et al. 2012; Jan et al. 2013; Liu et al. 2013). Several functio-
nal studies indeed demonstrated that aPKC
λ/ι
promotes tumor
progression. Constitutive active aPKC (aPKC
λ/ι
-CAAX) increased
intestinal tumor metastasis, whereas loss of aPKC
λ/ι
had the op-
posite effect (Murray et al. 2009). aPKC
λ/ι
in addition induced
invasive phenotypes of non-small cell lung cancer cells, where
aPKC-mediated phosphorylation of Par6 downstream of TGFß
receptor signaling promoted EMT, invasion and metastasis (Gu-
naratne et al. 2013; Regala et al. 2009). In gastric cancer, aPKC
expression correlated with lymphatic invasion and poor prog-
nosis (Yoshihama et al. 2013), and in esophageal cancer aPKC
may confer resistance to anoikis through activation of Akt-
dependent survival signaling (Liu et al. 2011). In hepatocellular
carcinoma, Par3 overexpression is a risk factor of extrahepatic
metastasis and associated with decreased 5-year survival (Jan
et al. 2013). As we had observed that Par3 in contacting ke-
ratinocytes serves to localize aPKC to intercellular adhesions,
and Par3 deficiency caused abnormal cytoplasmic localization
of aPKC, we wondered whether Par3 contributes to tumor pro-
gression
in vivo
. In DMBA/TPA-induced skin tumors, we indeed
found that loss of Par3 results in increased tumor cell invasion
into surrounding stroma, identifying Par3 as an invasion sup-
pressor (Iden et al. 2012). This was in line with findings in Dro-
sophila, where mutations in bazooka/Par3 facilitates metastasis
of Ras-induced tumors (Pagliarini and Xu, 2003). Interestingly,
two recent independent studies could confirm a role of Par3 as
metastasis suppressor in breast cancer (McCaffrey et al. 2012;
Xue et al. 2013). Together, despite a high context dependency in
primary tumorigenesis, impaired Par3/aPKC function seems to
prevent invasion and metastasis of various cancers. Underlying
mechanisms are likely diverse and may include alterations in
cohesion between cells, cell-ECM interactions, metabolic repro-
gramming, sensitivity towards micro-environmental measures,
cytoskeletal reorganization fueling cell motility, regulation of
survival and growth signaling outside the primary tumor niche,
and induction of anoikis.
Therapeutic outlook
Our animal model with epidermal Par3 deficiency represents a
clinically relevant system to study processes underlying kera-
toacanthoma formation and progression. Given the dual role
of Par3 in different skin cancers, direct therapeutic targeting
of Par3 for prevention of human cancer is not advised. First, a
better understanding of described context-dependencies is re-
quired. Importantly, however, therapeutic targeting of the pola-
rity machinery could occur via aPKC and perhaps another kinase
linked to the Par polarity network, the tumor suppressor AMPK.
A phase I dose escalation study of the aPKC
ι
inhibitor ATM for
treatment of advanced non-small-cell lung cancer, ovarian can-
cer, and pancreatic cancer has recently been completed success-
fully (Mansfield et al. 2013). Next to aPKC-mediated oncogenic
signaling towards Rac-PAK-MEK-ERK, which likely reflects the
predominant signaling axis in ATM-sensitive tumors, inhibition
of aPKC may also re-establish sensitivity towards chemothera-
py through induction of apoptosis (Murray et al. 2012; Rimessi
et al. 2012) or towards cellular senescence (Paget et al. 2012).
Inhibition of aPKC signaling may thus represent an important
concept for prevention and/or therapy of human cancer. Whe-
ther inhibition of aPKC is able to prevent epidermal tumors re-
mains to be tested.
Administration of metformin, a first-line anti-diabetic drug int-
roduced into the clinic several decades ago, activates AMPK and
is associated with reduced cancer incidence and increased life
span of patients. The tumor-suppressive potential of metformin
has been functionally confirmed in prostate cancer lines (Ben
Sahra et al. 2010) and
in vivo
in the two-stage skin carcinoge-
nesis model where metformin administration antagonized TPA-
mediated skin tumor growth (Checkley et al. 2013). Although it
is currently unclear whether AMPK and Par3/aPKC counteract
each other in skin cancer, these studies open interesting ave-
nues towards pharmacological targeting of polarity signaling in
human cancer.
Research news


