cell news 2/2013
5
binder innovation prize
thelial spreading during zebrafsh epiboly thus directly links
force generation of a single, albeit large, cellular structure with
morphogenetic shape changes of an entire epithelial tissue. We
will outline how a combination of experiments together with a
theoretical description of actomyosin network mechanics, has
provided insight into the biophysical mechanisms driving it (15).
Zebrafsh epiboly
Zebrafsh gastrulation is an excellent assay system for studying
the biophysical mechanisms of global tissue organization. Major
morphogenetic movements result in the formation of distinct
germ layers (ectoderm, mesoderm, endoderm) and the estab-
lishment of an embryonic body axis
(16)
during the course of
gastrulation taking place between 4 hours and 10 hours post
fertilization (hpf). Epiboly comprises a central gastrulation mo-
vements, referring to the thinning of the blastoderm as it engulfs
the underlying yolk cell in an animal-to-vegetal (AV) directed
spreading (Fig. 1). Three different types of tissues are involved in
this process. The deep cells, which will form the embryo proper
and are initially residing at the animal pole on top of the yolk
cell, and two extra-embryonic tissues - the yolk syncytial layer
(YSL), a thin multinucleated cytoplasmic layer at the surface of
the yolk sac, and the enveloping cell layer (EVL), a simple squa-
mous epithelial cell layer that covers the deep cells and is con-
nected at its margin with the YSL
(17)
. The molecular and cellu-
lar processes by which these different cell types/tissues undergo
epiboly movements have only begun to be understood
(18)
.
The initiation phase of epiboly, also referred to as ‘doming’ (4.33
hpf), is characterized by a drastic reorganization of the blas-
tomere mass, as the deep cells radial intercalate to fatten the
tissue. Simultaneous with the deep cell rearrangements, the yolk
cell underneath bulges towards the animal pole in a doming
movement
(19)
. Cell motility of the deep cells, recently shown
to depend on E-Cadherin endosomal traffcking
(20)
, is critical
in this process. Whether radial intercalation during doming is
driven by an active remodeling of the deep cells
(21)
or is rather
a passive consequence of pushing forces from the bulging yolk
cell
(22)
remains unknown. Importantly, for subsequent phases
of epiboly, EVL movements are independent from deep cell epi-
boly as E-Cadherin loss of function leads to an arrest of deep
cell epiboly at the equator of the embryo, while the EVL progres-
ses normally
(23)
; more than doubling its surface area over the
course of a few hours. What is the driving mechanism for this
massive epithelial spreading?
EVL
yolk
cell
YSL
yolk
EVL
deep cells
tight
junctions
epiboly
epiboly
animal (A)
actomyosin
microtubules
nuclei
vegetal (V)
a
A
V
b
EVL
YSL
EVL
YSL
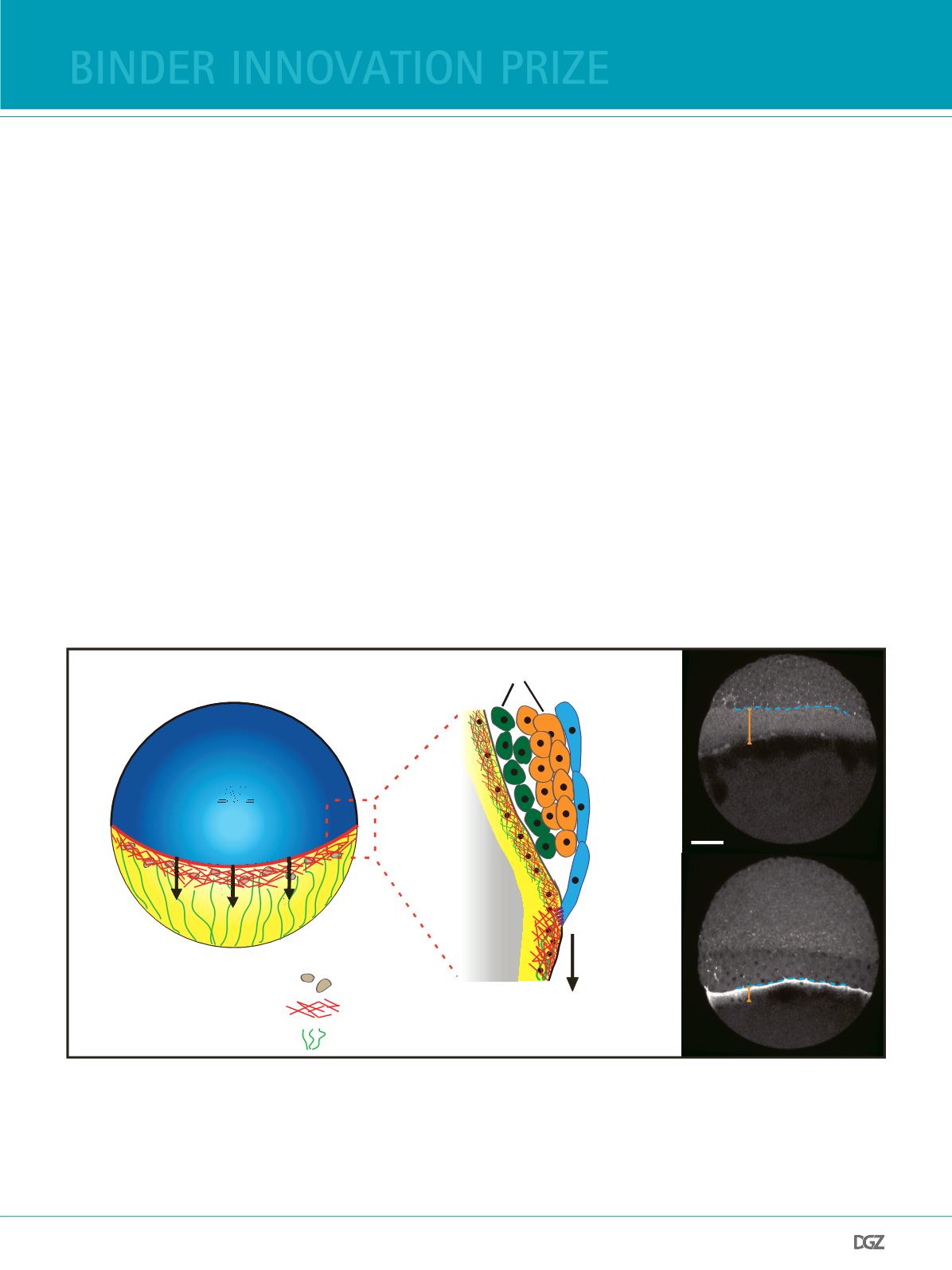
Figure 1:
Actomyosin ring formation during zebrafsh epiboly.
(a) (Left) A schematic depiction of a zebrafsh embryo at 50% epiboly. Epiboly movements refer to the animal-to-vegetal (AV) spreading of the tissues over
the yolk cell. (Right) Illustrative cross section of the tissues involved in epiboly. Deep cells give rise to the embryo proper and are enveloped by the EVL. The
EVL is connected at its margin to the YSL, a thin cytoplasmic layer containing nuclei and cytoskeletal structures.
(b) Myosin-2 localization in Tg(actb2:myl12.1-eGFP) embryos: At the onset of epiboly, a broad diffuse actomyosin band (orange bar) forms within the YSL
(40% epiboly, upper panel). As epiboly proceeds the band constricts to from a disctinct ring-like structure (70 % epiboly, lower panel). Scale bar, 50 µm.
Adapted from (15).


