cell news 2/2013
14
single-molecule reconstitution
of actin regulatory mechanisms
dennis breitsprecher
The actin cytoskeleton is an inherent part of
the eukaryotic cell. It not only provides me-
chanical support to maintain cell morpholo-
gy, but also mediates many dynamic cellular
processes. The rapid assembly and disassem-
bly of flamentous actin arrays – e.g. during
cytokinesis, vesicle traffcking, cell migration
or adhesion - is regulated by the interplay
of a large number of actin regulatory factors
which affect all aspects of flament dynamics,
ranging from nucleation and elongation over
bundling and capping to flament severing and
depolymerization.
A critical step at the onset of actin flament
dynamics is the
de novo
formation of fla-
ments, also called nucleation, which is media-
ted by nucleation factors (Chesarone & Goode,
2009). One family of nucleation factors, the
formins, is particularly interesting. Formins
not only promote the nucleation of new f-
laments, but also enhance their elongation
rates by processively tracking the fast gro-
wing “barbed” end while inserting actin mo-
nomers (Breitsprecher & Goode, 2013; Kovar,
2006; Pruyne et al, 2002; Romero et al, 2004).
Recently,
in vivo
and
in vitro
studies showed that collabora-
tions between formins and other nucleation promoting factors
(NPFs) are required in different organisms (Blanchoin & Miche-
lot, 2012). However, the underlying molecular mechanisms are
diffcult to elucidate in bulk experiments due to overlapping
functions of the proteins (e.g. nucleation of new flaments),
and thus where elusive for a long time.
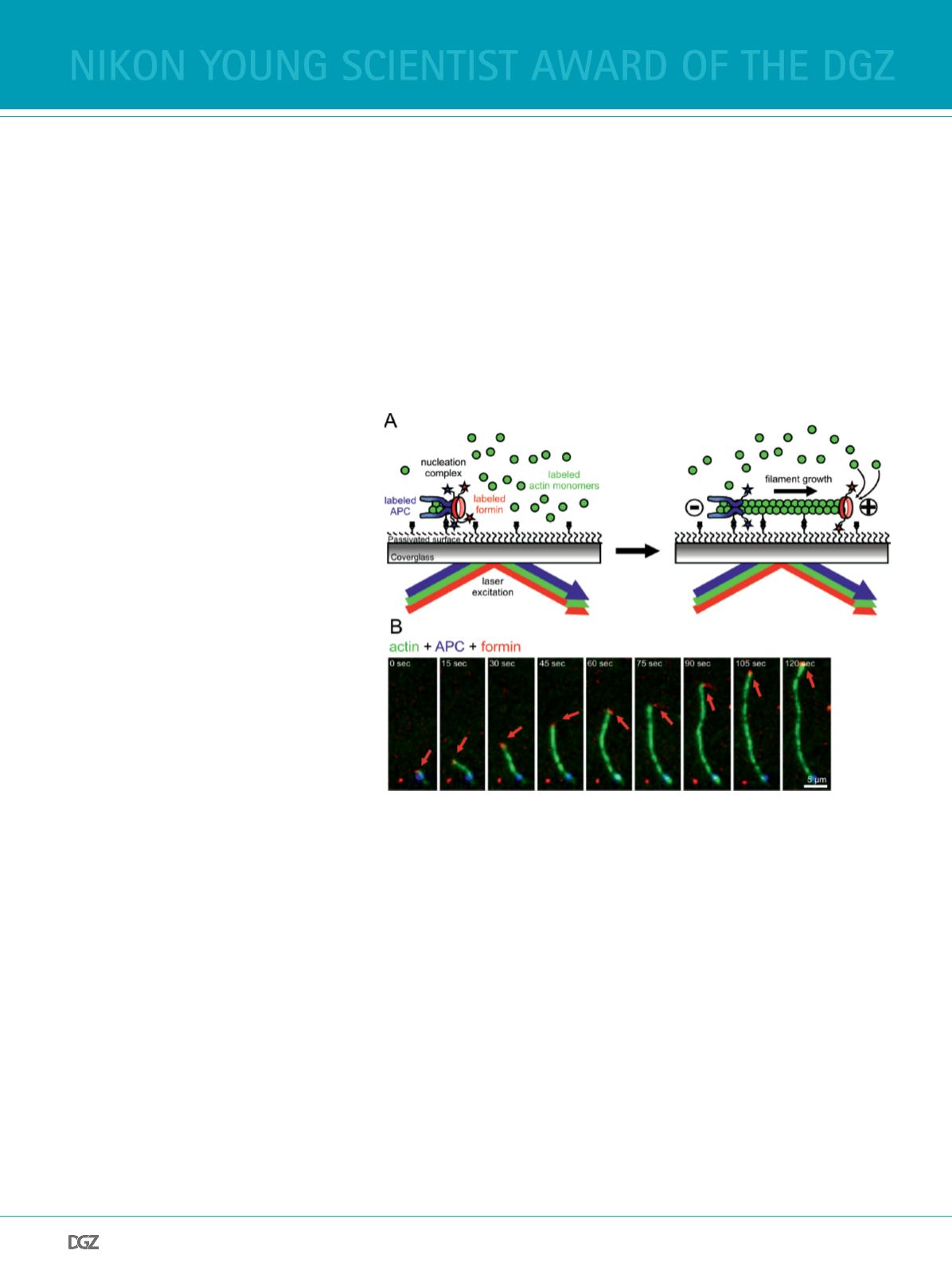
One elegant way to analyze such collaborations is to directly
visualize the interactions of the assembly factors
in vitro
on
the single molecule level. This can be achieved by multi-wave-
length total-internal-refection-fuorescence (TIRF) microsco-
py, which allows for the simultaneous imaging of multiple fu-
orescently tagged actin regulatory proteins and fuorescently
labeled actin flaments (Figure 1A). Experiments with tagged
variants of the formin mDia1 and the NPF adenomatous poly-
posis coli (APC) were instrumental to show that these proteins
form dimer:dimer complexes which bind actin monomers to
trigger effcient flament nucleation. After nucleation of the f-
lament, mDia1 and APC complexes separate driven by flament
growth, with the formin processively tracking the barbed end
while APC stays bound the site of nucleation (Figure 1A and B)
(Breitsprecher et al, 2012). Further experiments showed that
this mechanism was required for flament formation in the
presence of actin-assembly inhibitors, such as the sequestering
protein proflin and barbed end capping proteins. This examp-
le highlights the power of single-molecule imaging to dissect
complex multi-component mechanisms in a dynamic system.
Figure 1
Single molecule imaging of complex actin assembly mechanisms. A) Schematic representation
of APC/mDia1 collaboration. An actin flament is nucleated by the APC/mDia1 nucleation com-
plex and anchored to a passivated glass surface, with APC residing at the pointed end (-) while
mDia1 elongates the barbed end (+). B) Single-molecule TIRF micrographs of an actin flament
nucleated by APC-mDia1 collaboration and subsequently elongated by mDia1 (red arrow).
nikon young scientist award of the dgz


