cell news 2/2013
24
research news
internal lining of the heart tube. Both cell types are derived from
two bilateral felds of lateral plate mesoderm, from which they
frst initiate symmetrical movements towards the embryonic
midline (Trinh et al., 2004; Bussmann et al., 2007; Holtzmann
et al., 2007). In a second step, the cardiac cone reshapes and
breaks midline symmetry by typically extending towards the
left. This process, known as cardiac jogging, produces not only
L/R asymmetry but also results in the transformation of the fat
cardiac cone into an extending, hollow heart tube (Chen et al.,
1997; Rohr et al., 2008; Fig. 1).
The complexity of the morphogenetic rearrangement is appa-
rent when one considers the challenge of transforming a fat
epithelial sheet that is tightly constrained by neighboring tissu-
es into an elongated heart tube. The process depends on rapidly
changing and dynamic cellular behaviors. Within the heart cone,
myocardial cells exhibit strikingly different shapes depending on
their positions. Cells at the center, close to the midline, exhi-
bit columnar shapes, whereas cells at more lateral position
s are
cuboidal or even squamous (Trinh and Stainier, 2004). Hence,
myocardial cells apparently undergo a process of epithelial ma-
turation throughout the cardiac feld. In support of this obser-
vation, the loss of atypical protein kinase C iota (aPKCi) or of
Membrane protein palmitoylated 5 (Mpp5), two components
of the Partition (Par) and Crumbs protein complexes of cell
polarity regulators, completely abolishes heart tube formation
(Horne-Badovinac et al., 2001; Peterson et al., 2001; Rohr et
al., 2006; Rohr et al., 2008). Thus, early heart tube formation
is an epithelial tissue transformation process that requires that
cardiac progenitor cells must be organized as a highly polarized
epithelial tissue.
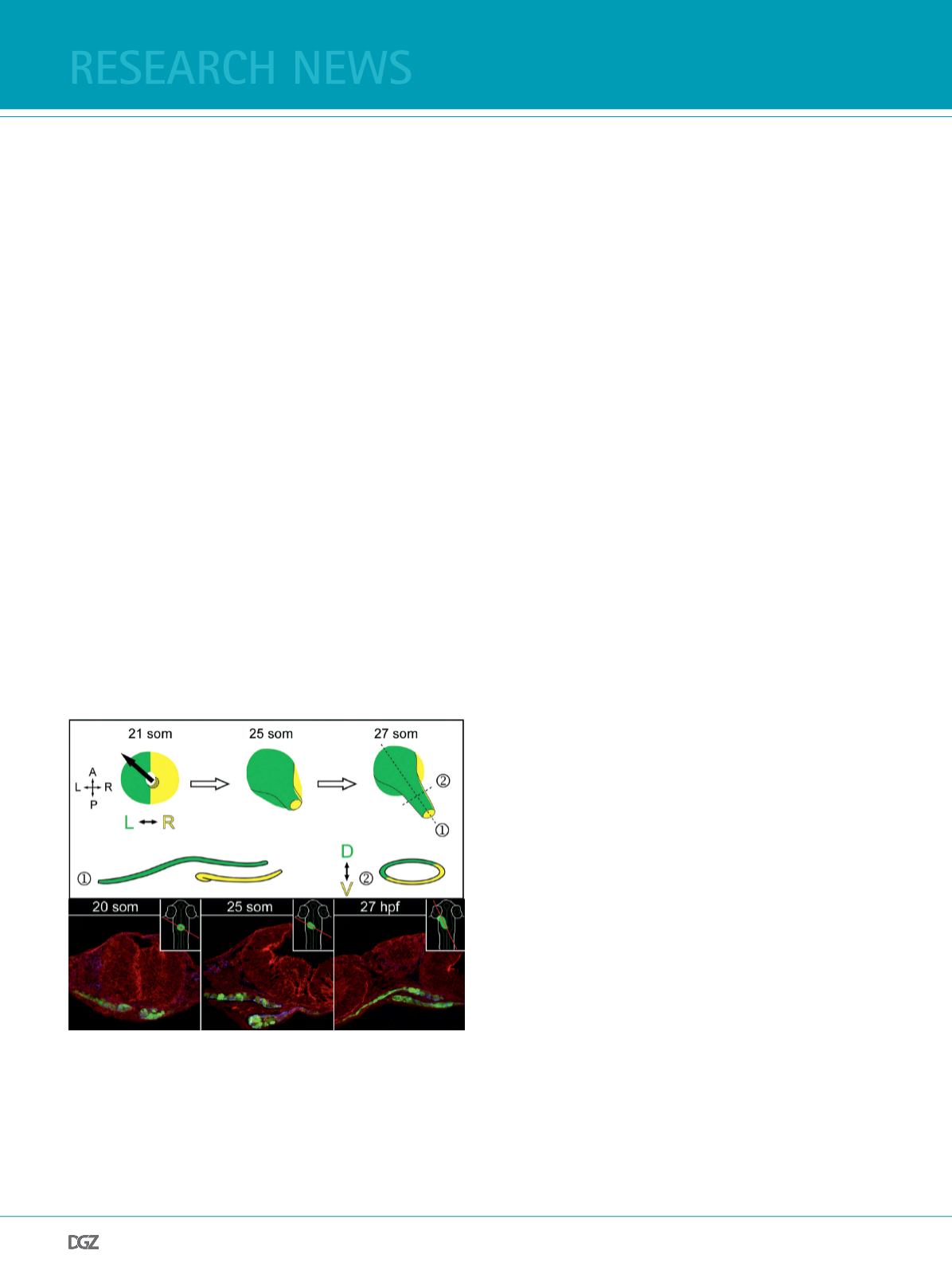
Cardiac tube formation commences with the occurrence of an
involution fold of myocardial cells that are within the right
half of the cardiac cone and move ventrally to form the ventral
foor. Left-sided myocardial cells, on the other hand, do not
involute; instead they establish the dorsal roof of the nascent
heart tube. This intricate process generates the frst morpho-
logical asymmetry in the zebrafsh embryo and progressively
transforms the fat cardiac cone into the heart tube (Rohr et al.,
2008; Fig. 1). By the end of the transformation process, endocar-
dial cells that were initially positioned below the cardiac cone
are included within the heart tube (Bussmann et al., 2007). Live
confocal microscopy revealed that left-sided myocardial and
endocardial cells move with higher velocities than those on the
right (Baker et al., 2008; de Campos-Baptista et al., 2008; Rohr
et al., 2008; Smith et al., 2008; Lenhart et al., 2013; Veerkamp
et al., 2013). The fact that L/R asymmetry of the heart depends
on the Nodal co-receptor One-eyed pinhead, left-sided Nodal
ligand Southpaw (Spaw)/Nodal-related 3 (Ndr3)(which is refer-
red to as Spaw), and Bmps raised the intriguing possibility that
the L/R asymmetric behavior of cardiac cells may be under the
direct control of Nodal and Bmp signaling. The functional relati-
onship of Nodals and Bmps, the consequences of complex TGFß
signaling for cellular behaviors, and the target genes involved
in the execution of this process were largely unknown and have
become the focus of intensive studies.
nodal negatively modulates bmp activity by unilaterally
biasing the extracellular matrix composition
Among the great unresolved questions of L/R asymmetry are the
mechanisms by which Nodal signaling, once established, infu-
ences the cellular behaviors that underlie heart morphogenesis
(or, for that matter, morphogenesis of other midline organs such
as the gut). A number of excellent studies have reviewed the
mechanisms by which a L/R asymmetry of Nodal signaling is
initiated in vertebrates and will not be discussed here. In the
zebrafsh embryo, the L/R asymmetry of visceral organs depends
on the Nodal ligand Spaw, one of three Nodal ligands present in
that organism (Long et al., 2003). Spaw is exclusively expressed
on the left side of the embryo at some distance from the cardiac
cone (Fig. 2A). Within the left cardiac feld, Spaw activates se-
veral target genes, most likely due to long-range diffusion. That
Spaw does not activate target gene expression within the right
cardiac feld has been attributed to the expression of the secre-
ted Nodal antagonist Lefty1 at the embryonic midline; it acts as
an effcient barrier against the diffusion of Spaw (Lenhart et al.,
2011; Smith et al., 2011). The other important morphogen cas-
cade, the Bmp signaling pathway (Fig. 2B), has a strikingly re-
Figure 1:
Top row shows a schematic representation of the heart cone-to-tube
transition. The left side of the cardiac cone (green) contributes to the
dorsal roof whereas the right side (yellow) will form the ventral foor
of the nascent heart tube. The dorsoventral axis of the heart tube at
the 27-somite (som) stage is depicted in longitudinal (1) and transverse
sections (2). Below, cross sections through the heart feld in myocardial
reporter Tg(
myl7:EGFP
)
twu34
transgenic embryos (myocardial cells marked
green; F-actin, red) show the progressive formation of the nascent heart
tube (Rohr et al., 2008).


