cell news 2/2013
21
research news
actin 190 nm) forming the ring canal as previously observed
using electron microscopy (Tilney et al., 1996). Thicker actin fla-
ment bundles (> 229 nm) form a basket around the ring canals
and thinner cytoplasmic actin flament bundles (103-230 nm;
measurements in fgure 5D) extend from the cortex to the nuclei.
Loss of of the Arp2/3 or WRC function results in smaller and
abnormally shaped eggs (Zallen et al., 2002, Hudson & Cooley,
2002a). Similar phenotypes are observed in fies lacking mater-
nal Abi protein, an integral subunit of the WAVE complex. Ring
canals of abi mutant egg chambers are smaller than in wild-type
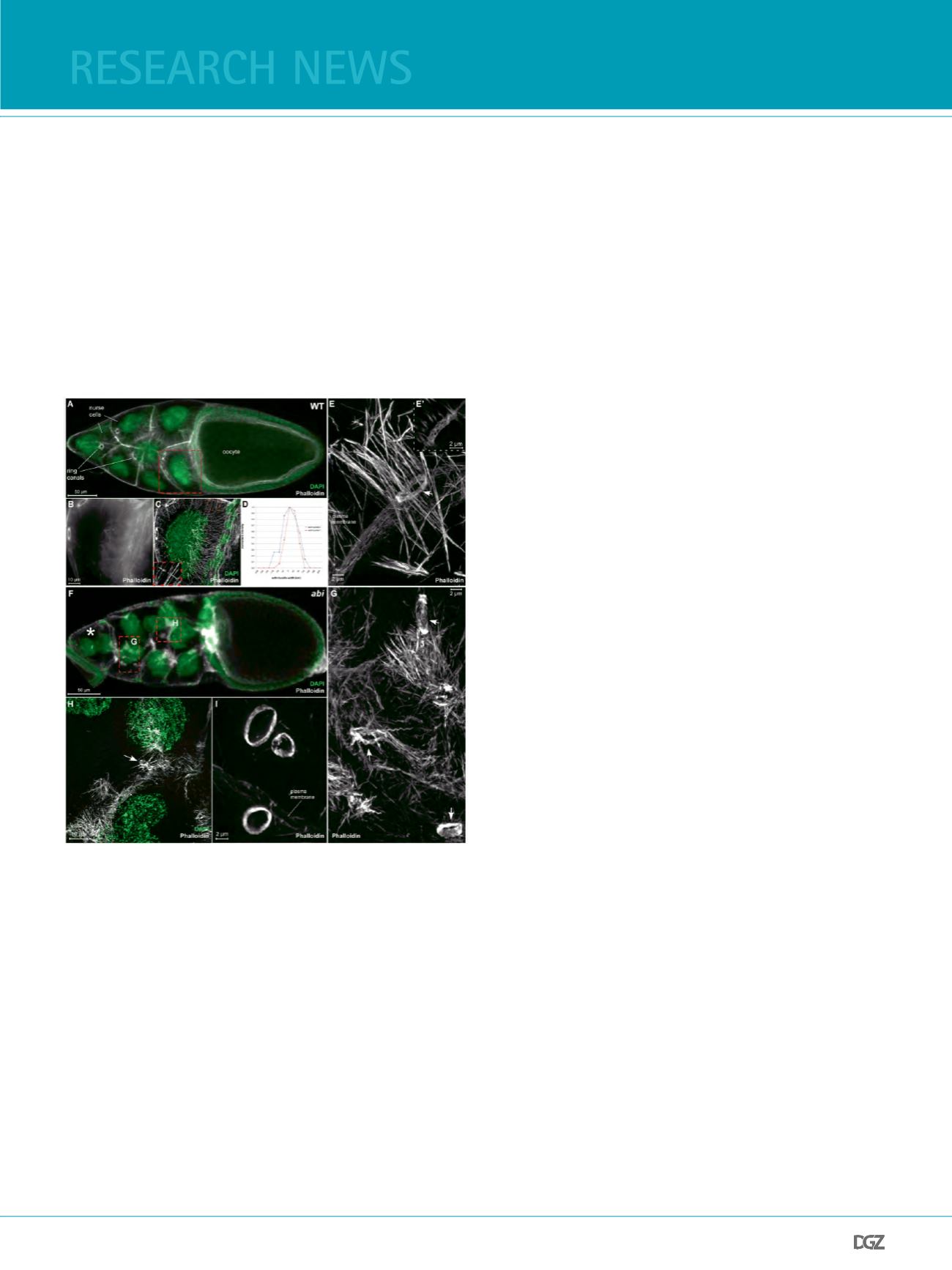
Figure 5: Fig. 5. 3D-SIM imaging of actin structures in Drosophila egg
chambers
(A) Conventional confocal image (LSM 510, Zeiss) of an early wild type
Drosophila stage 10B egg chamber stained with Alexa488 phalloidin to
reveal actin structures (white), and DAPI to visualize nuclei (green). Actin
structures such as ring canals, polyploid nurse cells and epithelial follicle
cells surrounding the oocytes are apparent. (B) Maximal intensity projection
of a conventional confocal image (LSM 510, Zeiss), (C) a 3D-SIM image of
the boxed region (red) in A. Cytoplasmic actin flament bundles extend from
the nurse cell cortex. (D) Plots of intensity along respective lines (#2 and
#7) in C. The apparent full width at half maximum (FWHM) was measured.
(E) 3D-SIM reconstruction of a ring canal (arrow) surrounded by a basket of
actin flament bundles. (E’) Single image of the 3D-SIM reconstruction in (E)
showing loosely packed flamentous actin. (F) Conventional confocal image
(LSM 510, Zeiss) of an abi mutant 10B egg chamber stained with phalloidin
(white) and DAPI (green). Subcortical F-actin became destabilized resulting
in multinucleated nurse cells (asteriks), (G, H) 3D-SIM images of the boxed
regions (red) in F. Ring canals of mutant egg chambers are often aberrantly
shaped (arrows in G, H), while cytoplasmic bundles are highly disorganized
probably due to membrane destabilization. (I) 3D-SIM image of ring canals
detached from the membrane. Scale bars are shown. Images taken from
Zobel and Bogdan, 2013.
and often aberrantly shaped (Zobel and Bogdan, 2013). Like in
later stage scar mutant egg chambers, ring canals are often de-
tached from the nurse cell membranes (Figure 5G-I, arrows) and
the subcortical actin becomes destabilized resulting in multinuc-
leated cells in abi mutants. Cytoplasmic bundles are still present
in mutant nurse cells, although they are highly disorganized pro-
bably due to membrane destabilization (Fig. 4 G). Thus, WAVE-
Arp2/3 mediated actin polymerization does not seem necessary
for the initial formation of these actin structures but rather im-
portant for their maintenance and for membrane integrity.
Concluding remarks
The enhanced resolution of the SIM technique now opens up
the perspective to revisit “old friends” as well as to identify new
candidates controlling the organization of the actin cytoske-
leton at the single cellular and multicellular level. Genetically
traceable model systems such as Drosophila egg chambers or
macrophages (called hemocytes) further allow researchers to
combine genetics with new advanced high resolution micro-
scopy techniques and live cell imaging in order to identify and
characterize the conserved regulatory network controlling cell
shape and cell motility in vivo.
Acknowledgement
I would like to thank all present and past members of lab. The work in my lab is currently funded
by the DFG (BO 1890/1-2, 2-1), the priority programme “Actin nucleators’ (SPP1464) and the
Cluster of Excellence “Cells in Motion“ (CIM).
Email:
website:
References
Bastock, R. & St Johnston, D. (2008) Drosophila oogenesis. Curr Biol, 18, R1082-1087.
Ben-Yaacov, S., Le Borgne, R., Abramson, I., Schweisguth, F., and Schejter, E.D. (2001). Wasp, the
Drosophila Wiskott-Aldrich syndrome gene homologue, is required for cell fate decisions media-
ted by Notch signaling. J Cell Biol 152, 1-13.
Bogdan, S. & Klambt, C. (2003) Kette regulates actin dynamics and genetically interacts with
Wave and Wasp. Development, 130, 4427-4437.
Bogdan, S., Grewe, O., Strunk, M., Mertens, A., and Klambt, C. (2004). Sra-1 interacts with Kette
and Wasp and is required for neuronal and bristle development in Drosophila. Development 131,
981-3989.
Bogdan, S., Stephan, R., Lobke, C., Mertens, A. & Klambt, C. (2005) Abi activates WASP to promote
sensory organ development. Nat Cell Biol, 7, 977-984.
Campellone, K.G., Webb, N.J., Znameroski, E.A., and Welch, M.D. (2008). WHAMM is an Arp2/3
complex activator that binds microtubules and functions in ER to Golgi transport. Cell 134,
148-161.
Campellone, K. G. & Welch, M. D. (2010) A nucleator arms race: cellular control of actin assembly.
Nat Rev Mol Cell Biol, 11, 237-251.
Chen, Z., Borek, D., Padrick, S. B., Gomez, T. S., Metlagel, Z., Ismail, A. M., Umetani, J., Billadeau,
D. D., Otwinowski, Z. & Rosen, M. K. (2010). Structure and control of the actin regulatory WAVE
complex. Nature, 468, 533-538.
Chesarone, M. A. & Goode, B. L. (2009) Actin nucleation and elongation factors: mechanisms and
interplay. Curr Opin Cell Biol, 21, 28-37.
Derivery, E. & Gautreau, A. (2010) Generation of branched actin networks: assembly and regula-
tion of the N-WASP and WAVE molecular machines. Bioessays, 32, 119-131.
Derry, J.M., Ochs, H.D., and Francke, U. (1994). Isolation of a novel gene mutated in Wiskott-
Aldrich syndrome. Cell 79, following 922.
Eden, S., Rohatgi, R., Podtelejnikov, A. V., Mann, M., and Kirschner, M. W. (2002). Mechanism of
regulation of WAVE1-induced actin nucleation by Rac1 and Nck. Nature 418, 790-3.
Fricke, R., Gohl, C., Dharmalingam, E., Grevelhorster, A., Zahedi, B., Harden, N., Kessels, M., Qual-
mann, B. & Bogdan, S. (2009) Drosophila Cip4/Toca-1 integrates membrane traffcking and actin
dynamics through WASP and SCAR/WAVE. Curr Biol, 19, 1429-1437.


