cell news 2/2013
11
werner risau prize
i
Δ
EC
Control
Rbpj
a
Control
Vegfr2
i
Δ
EC
i
Δ
EC
120
100
80
60
40
20
120
100
80
60
40
20
Vegfr2
Dll4 Hey1
Pecam
Cdh5
Dll4 Hey1
Vegfr2
Lung RNA qRT-PCR
%
%
CDH5
P-VEGFR2
VEGFR2
Lung RNA qRT-PCR
Lung Protein
WB
Control
Rbpj
i
Δ
EC
Anti-VEGF (72h)
Anti-VEGF (72h)
Rbpj
i
Δ
EC
Control
Dll4
i
Δ
EC
Dll4/Vegfr2
i
Δ
EC
Vegfr2
i
Δ
EC
b
c
d
e
Figure 1
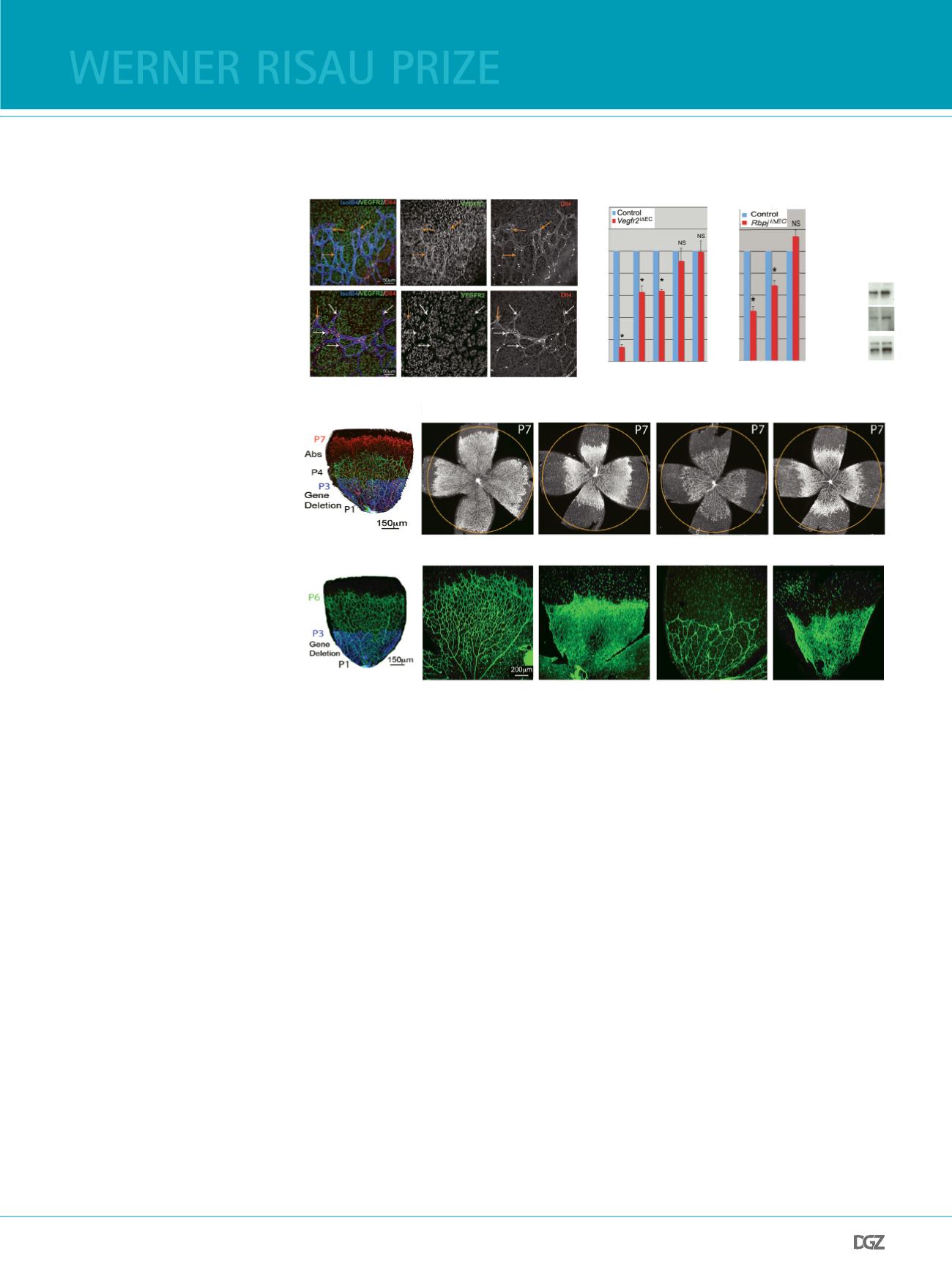
(a) Whole-mount triple immunofuore-
scence for Isolectin B4 (blue), Dll4 (red)
and VEGFR2 (green) of P6 control and
Vegfr2
i
Δ
EC
retinas. Deletion of
Vegfr2
for
5 days strongly compromises angioge-
nesis but not Dll4 expression. Arrows
indicate endothelial cells with (orange)
and devoid of VEGFR2 (white).
(b) qRT-PCR analysis of
Vegfr2
i
Δ
EC
and
Rbpj
i
Δ
EC
P6 mouse lungs for the indi-
cated transcripts showing that there is
Dll4
and
Hey1
expression in endothelial
cells with
Vegfr2
deletion and that there
is no signifcant difference in
Vegfr2
expression in endothelial cells after
impairment of the Notch downstream
transcriptional activity (
Rbpj
i
Δ
EC
). Error
bars represent s.e.m.; Asterisk, P <
0.001; NS, not statistically signifcant.
(c) Western blot analysis of
Rbpj
i
Δ
EC
P6
mouse lungs for the indicated proteins
showing no signifcant difference in
total or active VEGFR2 in these mutants.
VE-cadherin (Cdh5) protein is not re-
gulated by Notch and refects the total
endothelial content of both samples.
(d) Isolectin B4–stained control and
Rbpj
i
Δ
EC
P7 retinas (tamoxifen admi-
nistration from P1 to P3) treated with
control IgG or anti–VEGF-A antibodies
from P4 to P7 (72 hours). Orange
circles facilitate comparison of vascular
progression.
(e) Induction of EC–specifc
Dll4
or
Vegfr2
deletion from P1 to P3 and analysis at P6. Angiogenesis in
Dll4/Vegfr2
i
Δ
EC
and
Dll4
i
Δ
EC
mutants is strongly enhan-
ced compared to control and
Vegfr2
i
Δ
EC
retinas suggesting that impairment of Notch signalling turns endothelial cells angiogenically active independently
of VEGF/VEGFR2 signalling.
the VEGF family, Vegfr3/Flt4
12,13
. In contrast to VEGFR2, this recep-
tor does not bind VEGF. It only binds VEGFC and VEGFD and these
ligands are known to be very important for lymphatic vascular
development but in most contexts they are not so important for
blood vessel development
14
, unlike the VEGF/VEGFR2 signalling
axis that is a very powerful regulator of blood vessel development.
For this reason VEGFR3 was regarded as a receptor with a minor
function in Notch regulated angiogenesis. However, and in cont-
rast to
Vegfc/Vegfd
double KO embryos that have no major defects
in early blood vessel development, Vegfr3
-/-
embryos die at E10.5
with vascular defects
15
, suggesting that VEGFR3 might also have
an important but canonical ligand-independent function during
angiogenesis that cannot be compensated by VEGFR2.
With this in mind we investigated in more detail how Notch
regulates Vegfr3 function
in vivo
. In contrast with some pre-
vious reports we did not detect a substantial increase in
Veg-
fr3
transcription after impairing Notch signalling specifcally
in endothelial cells undergoing angiogenesis (Fig. 2a). But we
did detect a strong upregulation of VEGFR3 protein levels and
signalling activity in these cells suggesting that the regulation
of VEGFR3 by Notch occurs mainly at the post-transcriptional
levels (Fig. 2b). We also found that both Notch and VEGF regu-
late VEGFR3 levels in a opposite and independent manner. VEGF
is normally required for VEGFR3 expression in angiogenic cells,
but inhibition of Notch in vessels that lost VEGF activity rescues
VEGFR3 levels (data not shown). To investigate the functional
consequence of the high levels and activity of VEGFR3 protein
in endothelial cells with low or compromised Notch signalling
we used a kinase inhibitor (MAZ51) that potently inhibits VEGF-
C- and VEGF-D-induced activation of VEGFR3 but only weakly
impairs VEGFR2 activation by VEGF-A
16
. Although treatment of
mice with MAZ51 alone, only weakly affected the number of
flopodia and sprouts at the angiogenic front of the control reti-
nal vasculature, this inhibitor strongly suppressed the enhanced
sprouting caused by DAPT (Fig. 2c, d). These results showed that
endothelial cells are normally not very sensitive to VEGFR3 kina-
se inhibition, whereas in the absence of Notch signalling VEGFR3
levels increase and in this context endothelial cells seem to be
much more sensitive to VEGFR3 activity in accordance with pre-
vious results obtained in zebrafsh where morpholinos or
Vegfr3
mutants were used
12,17
.


