cell news 2/2013
15
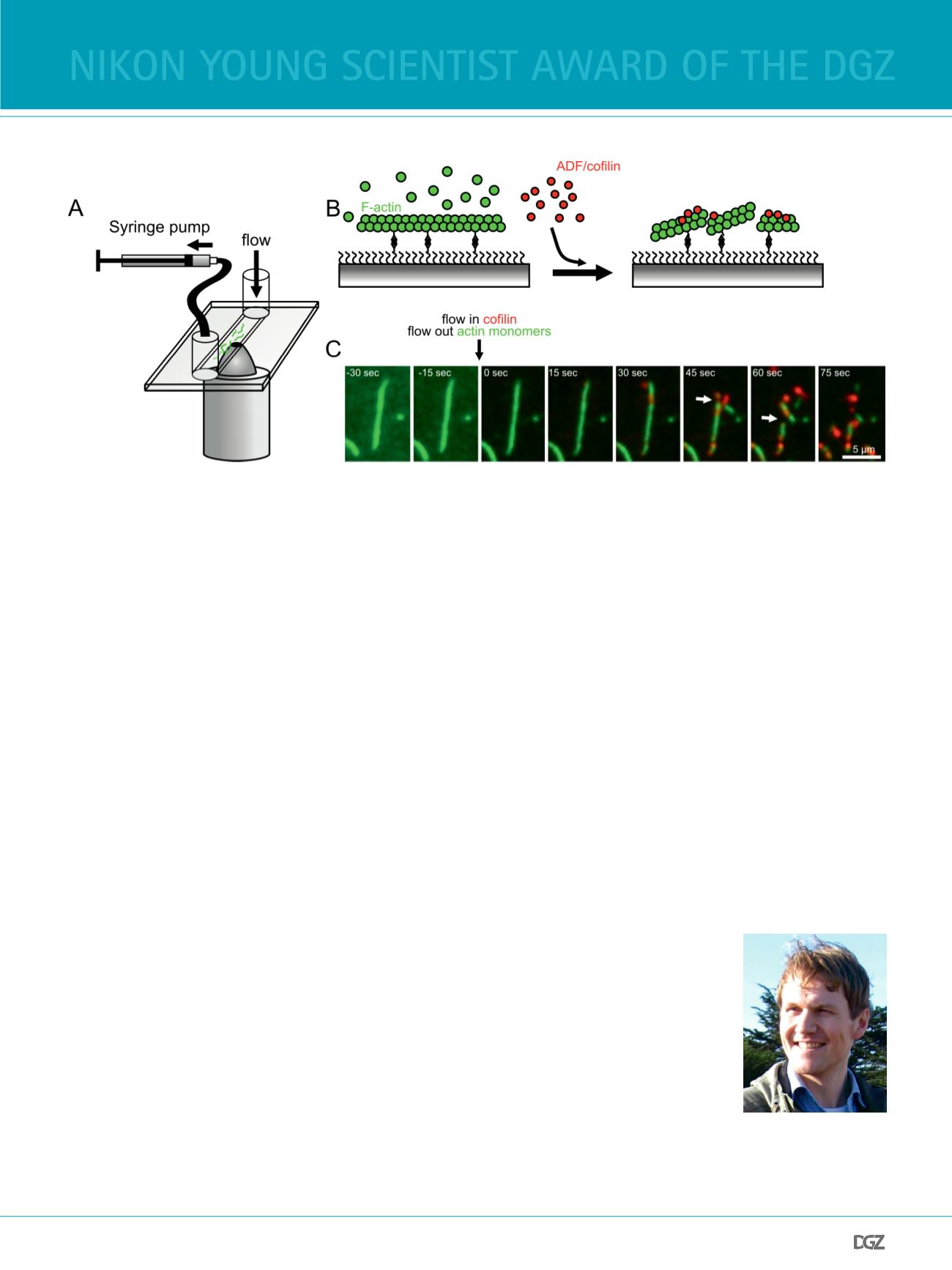
Figure 2
Reconstitution of actin disassembly. A) Schematic representation of a microfuidic chamber on an inverted TIRF-microscope objective. Flow can be induced
by pulling liquid through the fow chamber. B) Schematic representation of F-actin disassembly after addition of ADF/coflin. C) TIRF micrographs of actin
flament disassembly by fuorescently tagged coflin (red). Coflin is added at time 0, and the binding of coflin and subsequent severing of flaments
(arrows) can be observed over time.
In addition, single-molecule approaches are very versatile, and
can also be used to elucidate mechanisms of flament disas-
sembly and turnover, with just some minor modifcations of
the experimental design. The example in Figure 2A shows a
TIRF setup with a microfuidic fow chamber. Actin flaments
are frst polymerized in the fow chamber, and then monomers
are washed out and fuorescently tagged disassembly factors
are added to trigger depolymerization (Figure 2B). The major
actin flament disassembly factor in cells is the small F-actin
binding protein coflin. By using fuorescently tagged coflin
molecules in microfuidic experiments, accumulation of coflin
in distinct patches along the length of the actin flament can
be observed during depolymerization. Coflin binding eventu-
ally induces flament breaks, leading to flament fragmentation
(Figure 2C). These experiments showed that a coflin-binding
protein, the ubiquitous cyclase-associated protein (Srv2/CAP),
specifcally enhances coflin severing effciency, and that this
activity was required for flament disassembly and turnover in
cells (Chaudhry et al, 2013). Thus, flament disassembly pro-
cesses, just as flament assembly processes, are controlled by
interactions of their regulatory factors, and not by the activi-
ty of one single protein. We are just beginning to understand
the complex mechanisms that regulate highly diverse actin-
dependent processes in the cell. In the future, single-molecule
reconstitution approaches will likely play a major role in gai-
ning a comprehensive systems understanding of how the ac-
tin cytoskeleton is regulated on the molecular level (Mullins &
Hansen,
2013).
References
Blanchoin L, Michelot A (2012) Actin cytoskeleton: a team effort during actin assembly.
Curr Biol 22: R643-645
Breitsprecher D, Goode BL (2013) Formins at a glance. J Cell Sci 126: 1-7
Breitsprecher D, Jaiswal R, Bombardier JP, Gould CJ, Gelles J, Goode BL (2012) Rocket laun-
cher mechanism of collaborative actin assembly defned by single-molecule imaging. Sci-
ence 336: 1164-1168
Chaudhry F, Breitsprecher D, Little K, Sharov G, Sokolova O, Goode BL (2013) Srv2/cyclase-
associated protein forms hexameric shurikens that directly catalyze actin flament severing
by coflin. Mol Biol Cell 24: 31-41
Chesarone MA, Goode BL (2009) Actin nucleation and elongation factors: mechanisms and
interplay. Curr Opin Cell Biol 21: 28-37
Kovar DR (2006) Molecular details of formin-mediated actin assembly. Curr Opin Cell Biol
18: 11-17
Mullins RD, Hansen SD (2013) In vitro studies of actin flament and network dynamics. Curr
Opin Cell Biol 25: 6-13
Pruyne D, Evangelista M, Yang C, Bi E, Zigmond S, Bretscher A, Boone C (2002) Role of
formins in actin assembly: nucleation and barbed-end association. Science 297: 612-615
Romero S, Le Clainche C, Didry D, Egile C, Pantaloni D, Carlier MF (2004) Formin is a proces-
sive motor that requires proflin to accelerate actin assembly and associated ATP hydrolysis.
Cell 119: 419-429
Dennis Breitsprecher studied Bioche-
mistry at Leibniz University in Hannover,
Germany. He then joined the lab of Jan
Faix as a PhD student at Hannover Me-
dical School, where he started his re-
search on actin assembly factors of the
Ena/VASP family. For his post-doc, he
received a Research Fellowship from the
German Research Foundation (DFG), and
joined the lab of Bruce Goode at Brand-
eis University in Waltham, MA, where he
developed single-molecule approaches to
reconstitute actin regulatory mechanisms.
nikon young scientist award of the dgz


