cell news 2/2013
12
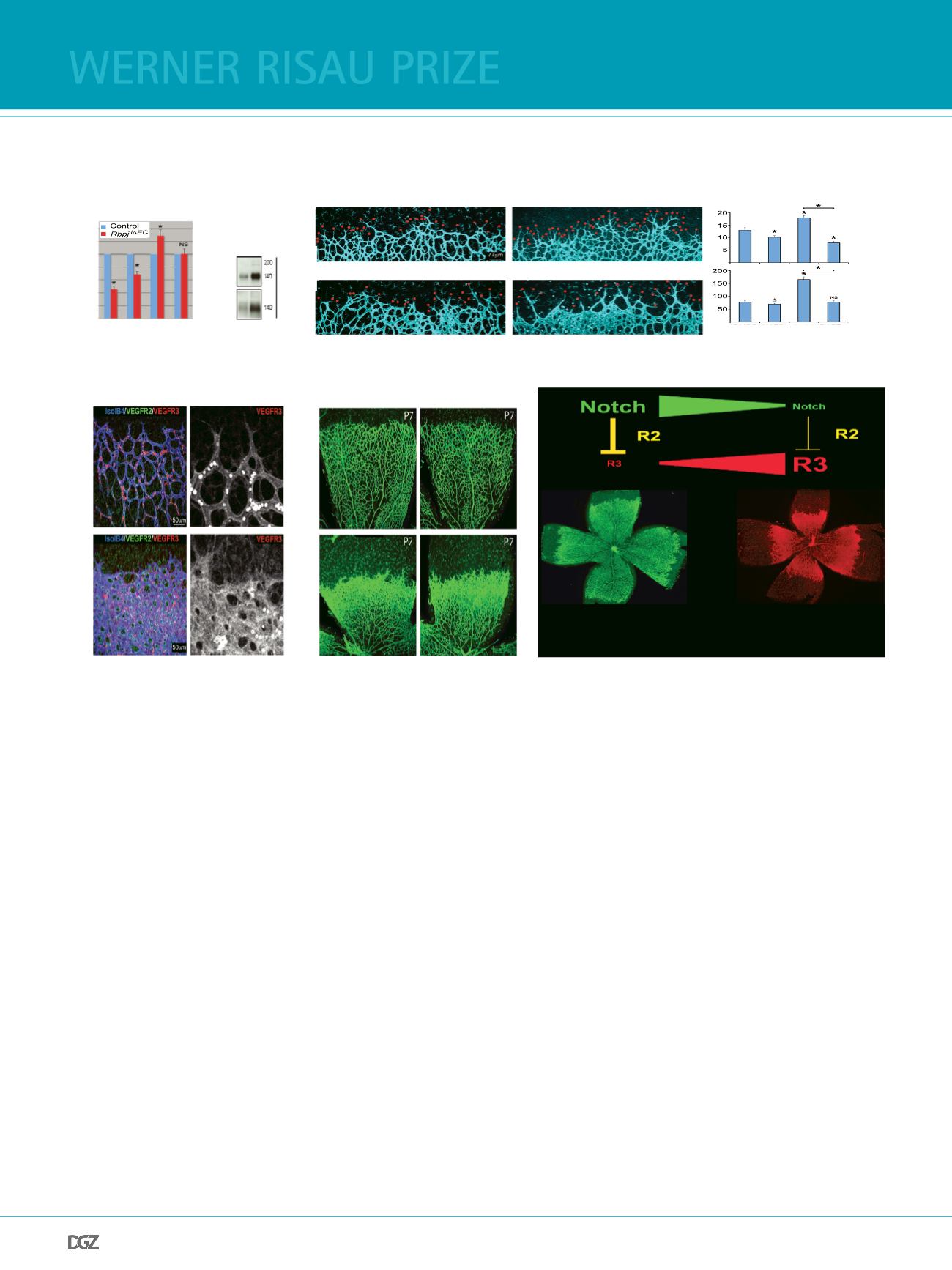
Figure 2
(a) qRT-PCR of
Rbpj
i
Δ
EC
post-natal day 6 (P6) mouse lungs for the indicated transcripts. On the right is shown the western blot result from lung lysates
of the same animals, showing strongly increased total and phospho-VEGFR3 in
Rbpj
i
Δ
EC
mutants. Error bars represent s.e.m.; Asterisk, P < 0.001; NS, not
statistically signifcant.
(b) Isolectin B4 (blue) combined with VEGFR2 (green) and VEGFR3 (red) staining of control and
Rbpj
i
Δ
EC
retinas.
Rbpj
i
Δ
EC
vessels have higher VEGFR3 protein
levels. Bright, small and round cells are autofuorescent circulating blood cells.
(c, d) Angiogenic front vessels of a P6 mouse retina stained for isolectin B4 (blue) after 24 hrs of treatment (P5 to P6) with the indicated inhibitors. MAZ51
effciently blocks sprouting (red dots) of DAPT-treated endothelial cells. Error bars represent s.e.m.; Asterisk, P < 0.05; NS, not statistically signifcant.
(e) Confocal images of isolectin B4–stained (green) control and
Rbpj
i
Δ
EC
retinas (tamoxifen administration from P1 to P3) of P7 mouse pups injected with
control IgG or anti–VEGFR3 antibodies from P4 to P7 (72 hours).
(f) Schematic model illustrating the main fndings. During angiogenesis, Notch activation in most endothelial cells downregulates VEGFR3, turning endo-
thelial cell activity dependent on VEGF and VEGFR2 signalling. In endothelial cells with very low Notch signalling, VEGFR3 levels increase signifcantly and
this leads to excessive and highly deregulated ligand-independent angiogenesis.
%
Vegfr3
Dll4
Hey1
Cdh5
120
100
80
60
40
20
140
Lung RNA qRT-PCR
%
Control
Rbpj
i
Δ
EC
Control
Rbpj
i
Δ
EC
Total
IP:pY
IB:VEGFR3
Control
Rbpj
i
Δ
EC
Vehicle
DAPT (24h)
MAZ51 (24h)
DAPT + MAZ51 (24h)
DAPT
Vehicle
MAZ51
DAPT+MAZ51
Sprouts per
length
Filopodia per
length
IgG (72h)
Anti-VEGFR3 (72h)
Normal
VEGF-dependent
Angiogenesis
Excessive
VEGF-independent
Angiogenesis
a
c
d
b
e
f
werner risau prize
Altogether our results suggest that inhibition of Notch might
switch blood vessel angiogenesis from a VEGF-A/VEGFR2 de-
pendent mode to a VEGF-C/D/VEGFR3 regulated mode. To
further test this hypothesis we used the blocking antibody mF4-
31C1
18
to block binding of VEGF-C/D ligands to VEGFR3 during
normal or Notch impaired angiogenesis. Contrary to the initial
prediction, we did not see any signifcant difference in endo-
thelial sprouting after the administration of this antibody in
control or Notch mutant mice (Fig. 2e), suggesting that VEGFR3
might be active even in the absence of its canonical ligands as
was also previously proposed
14,19,20
. Experiments performed by us
with cell lines
in vitro
showed that the kinase inhibitor MAZ51
can block both ligand-dependent and independent VEGFR3 si-
gnalling, whereas the blocking antibody mF4-31C1 only blocks
the ligand-induced signal (data not shown). We think that this
difference explains the different
in vivo
effects of these two
reagents and suggest that in the context of angiogenesis and
low Notch signalling, VEGFR3 is active even in the absence of
canonical ligand binding.
The sum of our fndings indicate that during angiogenesis, all
endothelial cells have Notch activity, although at relatively
different levels, and in this context VEGFR3 levels are low and
VEGF/VEGFR2 are the most important regulators of endothelial
sprouting. In cells with very low Notch signalling or when we
inhibit Notch, the situation changes, VEGFR3 protein levels in-
crease signifcantly and it becomes phosphorylated even in the
absence of the canonical ligands. We think that in this context
there is ligand-independent and therefore highly deregulated
endothelial sprouting (Fig. 2f), which mimics aspects of growth-


