cell news 2/2013
26
kinase (caMLCK) - one of the regulatory proteins that phospho-
rylate and activate NMII - the laterality of the entire cardiac
feld is affected. In the most extreme cases, such misexpression
clones even cause an inversion of cardiac laterality, or situs in-
versus. That such inversion phenotypes can occur even in the
presence of normal left-sided Nodal signaling has several im-
portant implications for our understanding of cardiac laterali-
ty. This fnding implies that complex organ morphogenesis can
be explained as the net sum of individual cell behaviors within
tightly coherent epithelial groups of cells. It also suggests that
the laterality of the entire organ is not strictly predetermined,
which would argue against the existence of left-sided guidance
cues for cardiac progenitor cells. Could the highly stereotypical
morphogenesis of cardiac form instead be explained by a random
motility gradient that drives laterality? Random motility gradi-
ent models have been used to describe the process of chicken
axis elongation (Bénanzéraf et al., 2010).
Mathematical modeling suggests that complex organ form and
cardiac laterality can be explained by slight differences in the
biomechanical properties of individual cells, as long as these
cells are coherently organized. Since the cardiac cone has an
epithelial character, motility differences of single progenitor
cells can infuence the entire group of cells and hence organ
laterality. We performed simulations of this process based on
Figure 3:
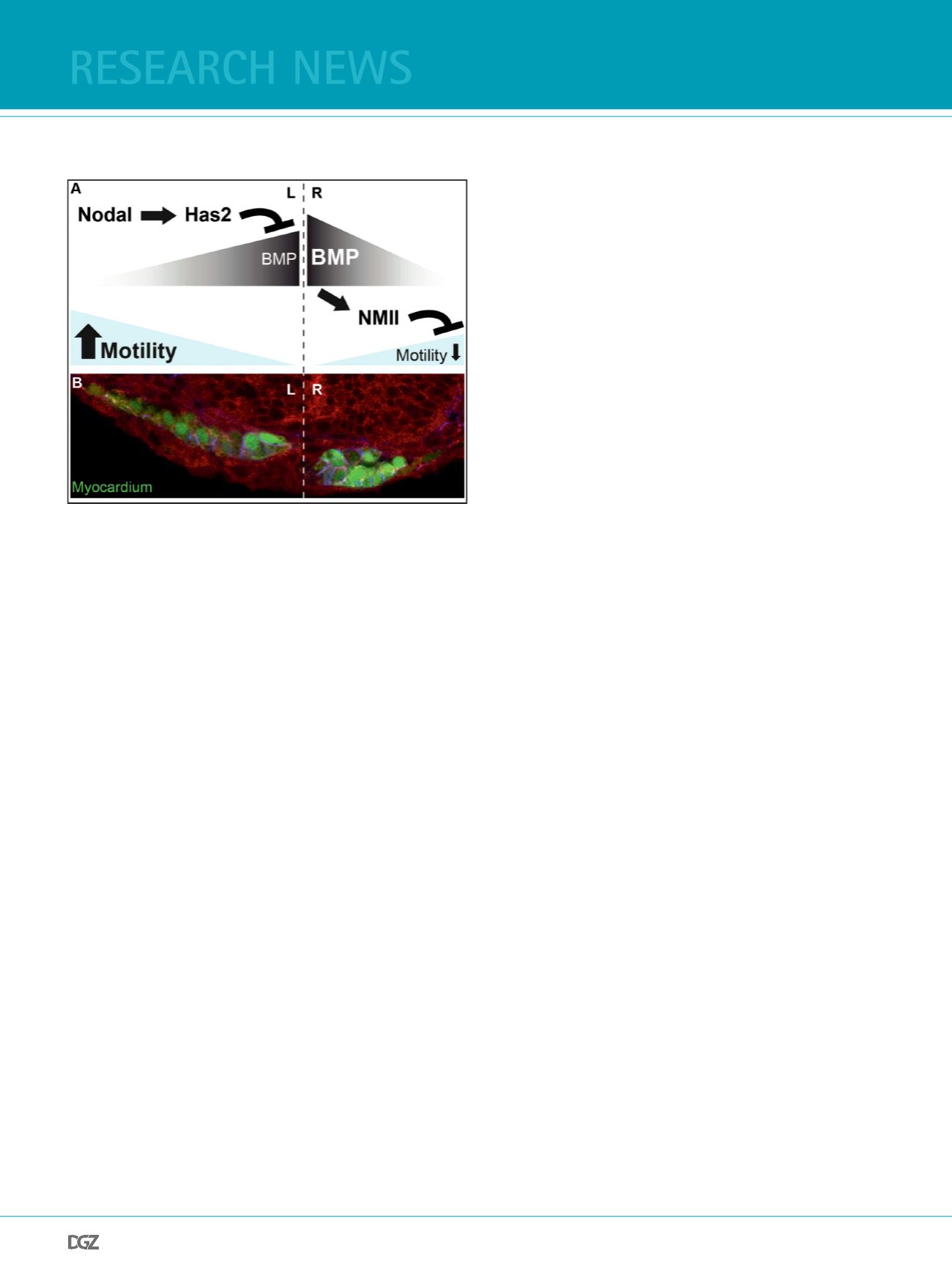
Cardiac laterality depends on Nodals and Bmps. (A) Schematic diagram
illustrating that the Nodal target Hyaluronan synthase 2 (Has2) dampens
Bmp activity within the left cardiac feld. Reduction of Bmp signaling on
the left causes lower expression of non-muscle myosin II (NMII) and higher
cardiac progenitor cell motility, which causes leftward directed asymme-
tric organ displacement. (B) Cross section through the cardiac cone in a
myocardial reporter Tg(
myl7:EGFP
)
twu34
transgenic embryo (myocardial cells
marked green; F-actin, red) shows L/R differences of myocardial morpholo-
gy (Veerkamp et al., 2013).
the assumption that cells on both sides of the embryonic midline
can freely and randomly move in any L/R direction, with cells on
the left side moving slightly faster. Invariantly, simulations using
these parameters resulted in a robust leftward displacement of
the coherent cardiac epithelium (Veerkamp et al. 2013). This mo-
del explains an apparent paradox: right-sided cardiac progenitor
cells that are not directly affected by Nodal still respond with
leftward motility. In principle, the faster rates of motility among
cardiac progenitor cells on the left can pull the entire cardiac
tissue in their direction. Thus individual, random cell motility is
the decisive force during the establishment of cardiac laterality
and can be more decisive than left-sided Nodal signaling.
conclusion
Our work outlines a novel mechanism by which Nodals and Bmps
regulate cardiac L/R asymmetry in zebrafsh. The principal me-
chanism involved in this process is an antimotogenic Bmp acti-
vity, which is negatively affected by Nodal. It comes as a great
surprise that such a well-choreographed and invariant organ
morphogenetic process is indeed based on a constant tug-of-
war between individual cardiac progenitor cells. This suggests
that some of the other wonderful structures that arise from
morphogenesis will also turn out to be the result of individual,
random cell behaviors rather than a predesigned blueprint.
Acknowledgements
I would like to thank all former and current members of my lab and Russ Hodge for their contri-
butions to our research and for continuous discussions of this topic. In particular, I would like to
thank Stefan Rohr and Justus Veerkamp who were the driving forces behind the work presented
in this review. I am currently supported by a Heisenberg Fellowship of the DFG.
References
Baker,K., Holtzman,N.G., and Burdine,R.D. (2008). Direct and indirect roles for Nodal signaling in
two axis conversions during asymmetric morphogenesis of the zebrafsh heart. Proc. Natl. Acad.
Sci. U. S. A 105, 13924-13929.
Bénazéraf,B., Francois,P., Baker,R.E., Denans,N., Little,C.D., and Pourquie,O. (2010). A random cell
motility gradient downstream of FGF controls elongation of an amniote embryo. Nature 466,
248-252.
Breckenridge,R.A., Mohun,T.J., and Amaya,E. (2001). A role for BMP signalling in heart looping
morphogenesis in Xenopus. Dev. Biol. 232, 191-203.
Bussmann, J., Bakkers, J., Schulte-Merker, S., 2007. Early endocardial morphogenesis requires
Scl/Tal1. PLoS Genet. 3, e140.
Chen,C.M., Norris,D., and Bhattacharya,S. (2010). Transcriptional control of left-right patterning
in cardiac development. Pediatr. Cardiol. 31, 371-377.
Chen,J.N., van Eeden,F.J., Warren,K.S., Chin,A., Nusslein-Volhard,C., Haffter,P., and Fishman,M.C.
(1997). Left-right pattern of cardiac BMP4 may drive asymmetry of the heart in zebrafsh. De-
velopment 124, 4373-4382.
Chocron,S., Verhoeven,M.C., Rentzsch,F., Hammerschmidt,M., and Bakkers,J. (2007). Zebrafsh
Bmp4 regulates left-right asymmetry at two distinct developmental time points. Dev. Biol. 305,
577-588.
Collery,R.F. and Link,B.A. (2011). Dynamic smad-mediated BMP signaling revealed through
transgenic zebrafsh. Dev. Dyn. 240, 712-722.
Conti,M.A. and Adelstein,R.S. (2008). Nonmuscle myosin II moves in new directions. J. Cell Sci.
121, 11-18.
de Campos-Baptista,M.I., Holtzman,N.G., Yelon,D., and Schier,A.F. (2008). Nodal signaling pro-
motes the speed and directional movement of cardiomyocytes in zebrafsh. Dev. Dyn. 237, 3624-
3633.
Holtzman, N. G., Schoenebeck, J. J., Tsai, H. J., Yelon, D., 2007. Endocardium is necessary for
cardiomyocyte movement during heart tube assembly. Development. 134, 2379-86.
Horne-Badovinac, S., Lin, D., Waldron, S., Schwarz, M., Mbamalu, G., Pawson, T., Jan, Y.N., Stai-
research news


