cell news 2/2013
29
research news
tingtin (Htt) protein (13). Mutation-based expansion of the CAG
stretch over a threshold of 37 consecutive codons increases the
propensity of Htt to aggregate in a CAG-length-dependent man-
ner (14). HD is associated with selective neuronal loss with the
highest vulnerability of the striatal neurons even though Htt
is ubiquitously expressed in the whole organism (15). The me-
chanism of specifc targeting of the striatial neurons remains
enigmatic. At later pathology stages, insoluble aggregates in
nucleus or cytoplasm of the disease-damaged tissue are built
(16). Whether the aggregation per se triggers pathology or rather
small soluble pre-aggregates cause cellular disfunction, is still a
matter of intense debate. Interestingly, along with the polyQ ag-
gregates in neuronal tissues, polyserine (polyS) and polyalanine
(polyA) species have been detected within the damaged neurons
of diseased individuals (17). The polyQ stretches are exclusively
encoded by the CAG codon even though another codon (CAA)
also codes for glutamine.
Translation of repetitive stretches may cause abnormal trans-
lation activities, including translation frameshifting. Translati-
onal frameshifting is a recoding event in which the ribosome
is forced to move to one of the alternative reading frames and
continuous to translate this frame instead of the original 0 frame
(18). Translational frameshift within the CAG repeat in +1 and -1
direction would result in AGC- and GCA-encoded stretches en-
coding for serine and alanine, respectively. Whether these polyS
and polyA species detected post mortem in the patients resul-
ted from a translational frameshift is still unknown. Thus, we
sought to investigate the frameshifting propensity of repetitive
CAG stretches within Htt exon 1. Indeed, expanded CAG stret-
ches are highly prone to frameshifting and the shift to -1 reading
frame (i.e., encoding polyAla) is more frequent (19). Performing
experiments to mechanistically understand the frameshifting
within expanded CAG stretches we came to a rather surprising
observation: the depletion of the cognate, charged glutaminyl-
tRNAGln-CUG is the main cause for -1 frameshifting within ex-
panded CAG repeats.
the amount of cag codons determines the frameshif-
ting frequency
To investigate the frequency of frameshifting, we used a reporter
system, in which the YFP gene is fused in -1 frame to Htt exon
1 with 51 CAG repeats (Figure 1A); -1 frameshifting will lead to
YFP expression. CAG-repeat expansion increases the susceptibi-
lity of Htt to intracellular proteases, releasing exon 1 comprising
the CAG repeat (20) which has much higher propensity to aggre-
gate and dominates the aggregates in the disease-damaged tis-
sues of patients (21). Thus, in our experiments we used only exon
1 with various CAG lengths. The reporter construct, Htt51Q(-1)
YFP, was ectopically expressed in murine neuroblastoma cell line
(N2a) stably expressing different CFP-tagged Htt constructs, Ht-
t65QCFP or Htt103QCFP (22). In all cells we detected YFP-posi-
tive species whose number was the highest in cells expressing
Htt103QYFP protein (Figure 1B). To our surprise, YFP-positive
spots reporting on frameshifted species appeared in the wild-
type N2a cells transfected only with the Htt51Q(-1)-YFP reporter
(Figure 1B). Importantly, the increased translation of CAG co-
dons in cells expressing Htt variants with longer CAG stretches
correlated with the frequency of frameshifting. The YFP-positive
species resulted from a frameshifting within the CAG stretch as
determined by mass spectrometry (Figure 1C).
Hybrid polyQ/polyA species with different Q:A ratio were for-
med (Figure 1C), suggesting that -1 frameshifting occurred sto-
chastically at any codon within the CAG repeat. This raised the
intriguing question as to whether the glutaminyl-tRNAGln-CUG
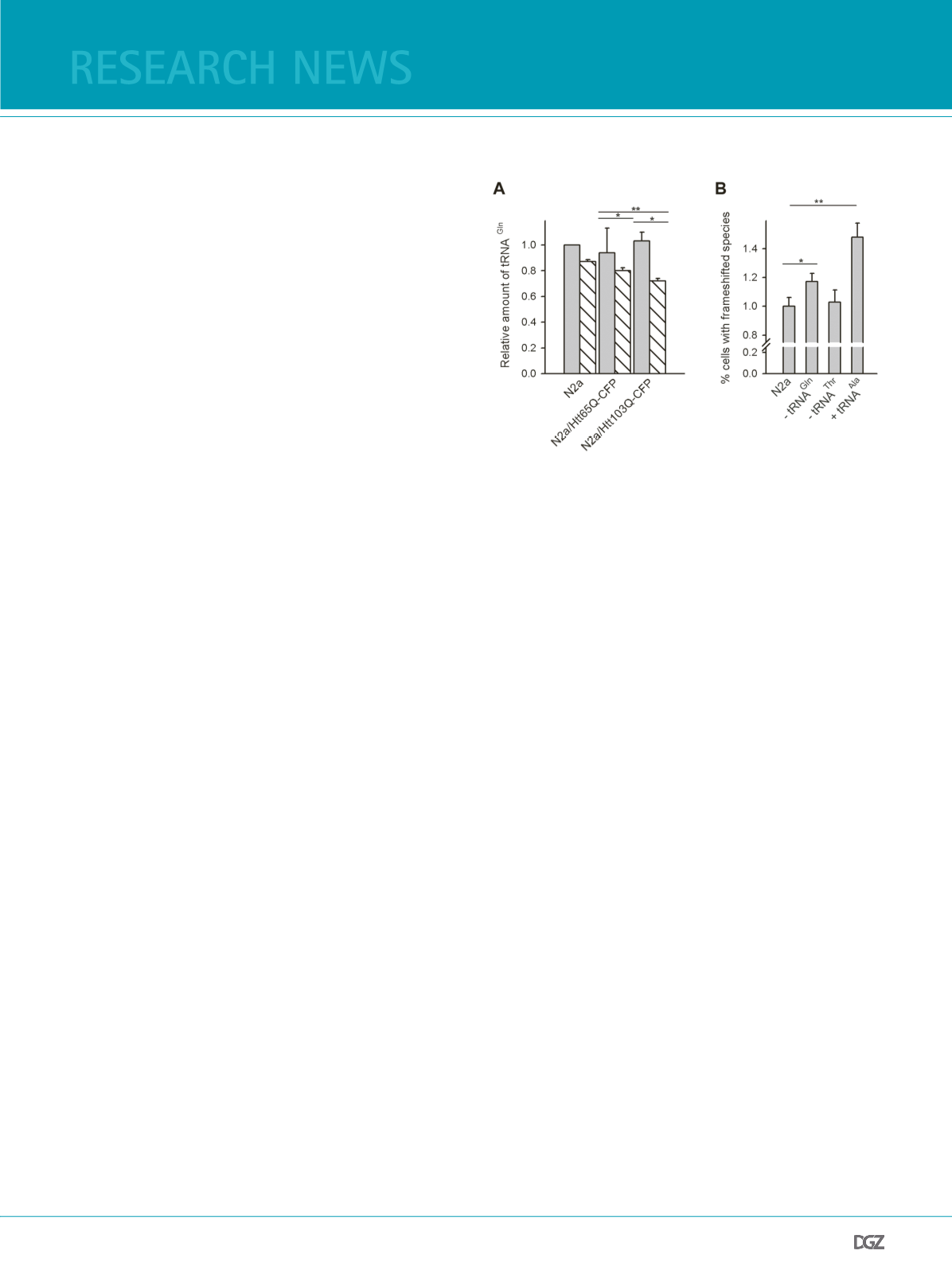
is depleted while translating long consecutive repeats. Measure-
ments of tRNAGln-CUG revealed no changes in the total concen-
tration of tRNAGln-CUG, however, a signifcant decrease of the
level of charged glutaminyl-tRNAGln-CUG (Figure 2A), implying
that an increased, simultaneous translation of CAG codons in
the cell reduces the concentration of translationally competent
aminoacylated tRNA. Furthermore, we decreased the tRNAGln-
CUG using the siRNA approach. Decrease of the tRNAGln-CUG
enhanced frameshifting (Figure 2B). This effect is specifc, as al-
Figure 2. Concentration of charged, glutaminyl-tRNAGln-CUG decrea-
ses in a CAG-length dependent manner:
(a) Total (gray bars) and aminoacylated-tRNAGln-CUG (dashed bars)
levels quantifed from the Northern blots of various N2a cells expressi-
on Htt51Q(-1)YFP reporter. The intensity of total tRNAGln-CUG of each
sample is normalized to the intensity of tRNAGln-CUG of the control N2a
cells. Glutaminyl-tRNAGln-CUG is determined as a fraction of the total
tRNAGln-CUG in each sample. Values are mean ± SD of 3 independent
experiments. * for p < 0.05, ** for p < 0.01.
(b) tRNAGln-CUG (- tRNAGln) and tRNAThr-AGU (-tRNAThr) were partially
silenced (appr. 40%) with siRNAs. tRNAAla-UGC was upregulated by
transfection with in vitro transcribed tRNAAla-UGC (+tRNAAla). The
frameshifting is represented as the percentage of cells (± SEM) containing
YFP-positive aggregates in the total population of cells transfected with
Htt51Q(-1)YFP (i.e., HA-positive) and compared to the control cells (N2a)
for which the percentage of frameshifted cells was set as 1 (as in Figure
1B). * for p < 0.05, ** for p < 0.01. Figure adopted from (19).


