25
Cell News 2/2015
that it is this core PCM layer, tightly associated with the cen-
triole wall, that provides a platform for further PCM growth
15,16
.
The recent high-resolution studies have revealed the molecular
composition of this “core PCM” layer to contain CPAP (Sas-4),
pericentrin and Cep152 (Asl). Interestingly, Sas-4 (
Drosophila
or-
tholog of CPAP) forms a cytoplasmic scaffold of S-CAP comple-
xes (Sas-4, Cnn, Asl and D-PLP) that constitutes core PCM layer
components. Functional studies suggest that in the absence of
Sas-4, nascent centrioles form but fail to mature into functional
centrosomes
17,18
. Overexpression of Sas-4 in flies produces PCM-
like structures
19
, while reduced amounts of Sas-4 in worms result
in centrosomes having proportionally less PCM
20
. Thus, these stu-
dies exclude the possibility that the identification of cytoplasmic
S-CAP scaffolds is a consequence of PCM disruption during uring
the experimental procedure
21
. Since the S-CAP components are
differentially recruited to centrosomes at various time points of
the cell cycle, the spatiotemporal assembly of cytoplasmic S-
CAP complexes and its contribution to overall amounts of PCM
recruitment remains to be elucidated.
PCM scales centrosome size and its MTOC activity
Although the MTOC activity of the centrosome is tightly correla-
ted with PCM size and cell cycle progression, the exact mecha-
nisms that regulate PCM assembly to control centrosome size
and capability remain largely unknown. Recent work by the Raff
laboratory elegantly identified that Polo-kinase (Plk1) phospho-
rylation of Cnn is required for PCM expansion during mitosis in
Drosophila
8,22
. Cnn recruitment in turn seems to be initiated by
another centrosomal protein, Asl
22,23
. Thus, Cnn molecules are
dynamically associated with the centrosome where they are first
recruited to the centriole proximity before spreading out to the
periphery. These studies suggest that Cnn is a major regulator of
PCM size in flies. On the other hand, PCNT has been shown to
regulate PCM expansion during centrosome maturation in hu-
man centrosomes. The Rhee laboratory identified certain amino
acid residues of the PCNT protein that can be phosphorylated
by polo-kinase to be crucial for centrosome maturation at the
onset of mitosis
24
. Using a variety of methods and a specific Plk1
inhibitor, they also demonstrated that the centrosomal recruit-
ment of Cep215 (human ortholog of Cnn) is independent of the
phosphorylation of PCNT. Instead Cep215-dependent
γ
-tubulin
recruitment that is essential for MTOC function of centrosomes,
requires phosphorylated PCNT during centrosome maturation
24
.
While these studies substantiate that Plk1 is a key kinase in trig-
gering centrosome maturation, its substrates seem to differ ac-
ross organisms, thus requiring further validation.
Aurora-A, a serine-threonine kinase, also plays a role in PCM
expansion at the onset of mitosis. The C-terminal portion of
Drosophila
Cnn directly interacts with Aurora-A and this inter-
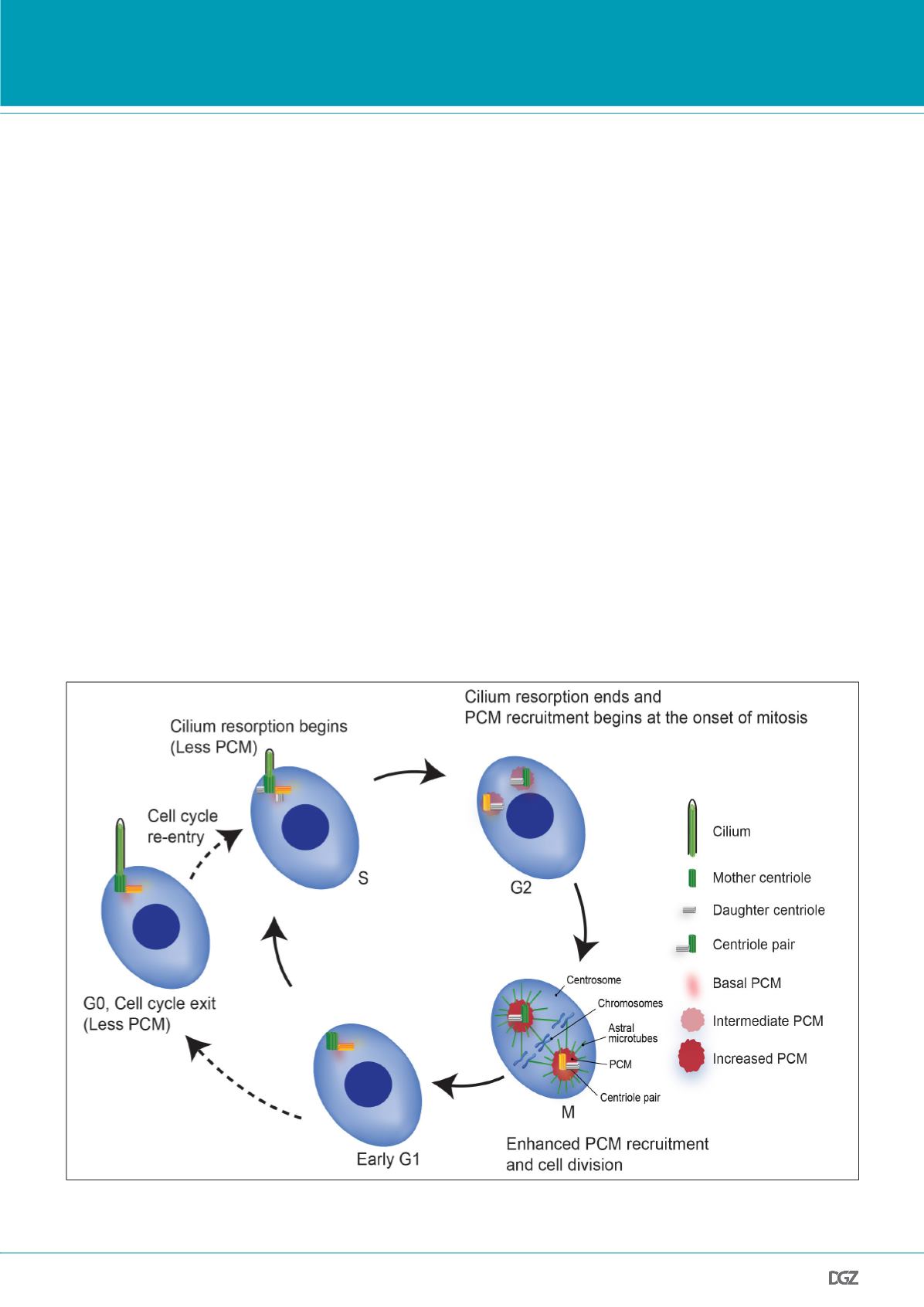
Figure 1. PCM dynamics in a cycling cell:
The mother centriole (green) with basal PCM (red) levels serves as a template for cilium formation during in-
terphase. Cilium disassembly at G2 triggers cells to continue with mitosis where centrosomes recruit more PCM to nucleate microtubules in the M phase.
RESEARCH NEWS


