19
Cell News 2/2015
WERNER RISAU PRIZE 2015
Mfsd2a
is critical for the formation and
function of the blood–brain barrier
Ayal Ben-Zvi
The central nervous system (CNS) requires a tightly controlled
environment free of toxins and pathogens to provide the proper
chemical composition for neural function
1
. Endothelial cells, the
building blocks of blood vessels of the brain, are considered to be
the gatekeepers of the brain. They protect the brain by blocking
entry or promoting clearance of harmful materials. They ensure
that the brain maintains the nutrient and chemical compositions
crucial for its unique metabolic demands and for neuronal func-
tion. Endothelial cells make up what is known as the blood brain
barrier (BBB). This barrier is very efficient in preventing entrance
of drugs/therapeutics from the blood to the brain and thus poses
a major obstacle for treating brain-related diseases. Conversely,
BBB breakdown is linked to degenerative pathologies, including
Alzheimer’s disease, ALS and Multiple Sclerosis
2
.
BBB endothelial cells display specialized tight junctions and ex-
tremely low rates of transcellular vesicular transport (transcyto-
sis)
3
. In concert with pericytes and astrocytes, this unique brain
endothelial physiological barrier seals the CNS and controls sub-
stance influx and efflux. BBB endothelial cells have lower rates
of transcytosis than endothelial cells in other organs
3
. Peripheral
endothelial cells display active vesicle trafficking to deliver nutri-
ents to peripheral tissues, whereas BBB endothelial cells express
transporters to selectively traffic nutrients across the BBB
4
. How-
ever, it is not clear when and how these properties are acquired.
Although recent studies revealed molecular pathways involved in
the development of the embryonic BBB
5–12
, disruption of some
of these genes affect vascular network development, making it
difficult to determine whether barrier defects are primary or se-
condary to a broader vascular effect.
A limited understanding of the molecular mechanisms that con-
trol BBB formation and BBB function has hindered our ability
to manipulate the BBB in disease and therapy
13
. We and others
showed that the BBB becomes functional during embryogenesis
in a gradual process of endothelial differentiation. In our current
work
14
, we showed that examining barrier-genesis at the mole-
leaked outside the vessels in
Mfsd2a
2
/
2
embryonic brains andwas found
in the cortical parenchyma (Fig. 4a) and individual parenchyma cells
(quantified as tracer-positive parenchyma cells per unit area of the de-
veloping lateral cortical plate; Fig. 4b). Furthermore, using imaging and
spectrophotometric quantification methods
5
, we found that the leaky
phenotype persisted in early postnatal (Extended Data Fig. 4) and adult
(Fig. 4c)
Mfsd2a
2
/
2
mice. Because the sequence of Mfsd2a has simi-
larities to the major facilitator superfamily of transporters, andMfsd2a
facilitates the transport of tunicamycin in cancer cell lines
23
, we injected
two non-carbohydrate-based tracers of different sizes to rule out the pos-
sibility that dextran leakiness is due to interactions withMfsd2a. Sulfo-
NHS-biotin (
,
550 Da) and horseradish peroxidase (HRP;
,
44 kDa)
tracers exhibited the leaky phenotype in
Mfsd2a
2
/
2
mice (ExtendedData
Fig. 4a, b). Moreover, a larger molecular weight tracer, 70-kDa dextran,
also displayed leakiness in
Mfsd2a
2
/
2
mice (Extended Data Fig. 4d).
In contrast to severe barrier leakage defects (Fig. 4a–c and Extended
Data Fig. 4), brain vascular patterning was similar between
Mfsd2a
2
/
2
mice and littermate controls. No abnormalities were identified in capil-
lary density, capillary diameter or vascular branching (Fig. 4d and Ex-
tended Data Fig. 5a), in embryonic (E15.5), postnatal (P4), and adult
(P70) brains of
Mfsd2a
2
/
2
mice. Moreover, we found no abnormalities
in cortical arterial distribution in adult
Mfsd2a
2
/
2
mice (ExtendedData
Fig. 5b).
tion of a f
This resu
cular ing
demonst
tinct pro
We ne
formatio
tron mic
nous HR
abnorma
At E17.5,
normal,
where a
microgra
was reve
lumen. I
cellular s
distances
between
tight jun
mice dis
luminal
cytoplas
tosis (Fig
lumen-c
Greater t
compare
along the
thermore
10-kDa tracer
Lectin
Overlay
E13.5
E14.5
E15.5
b
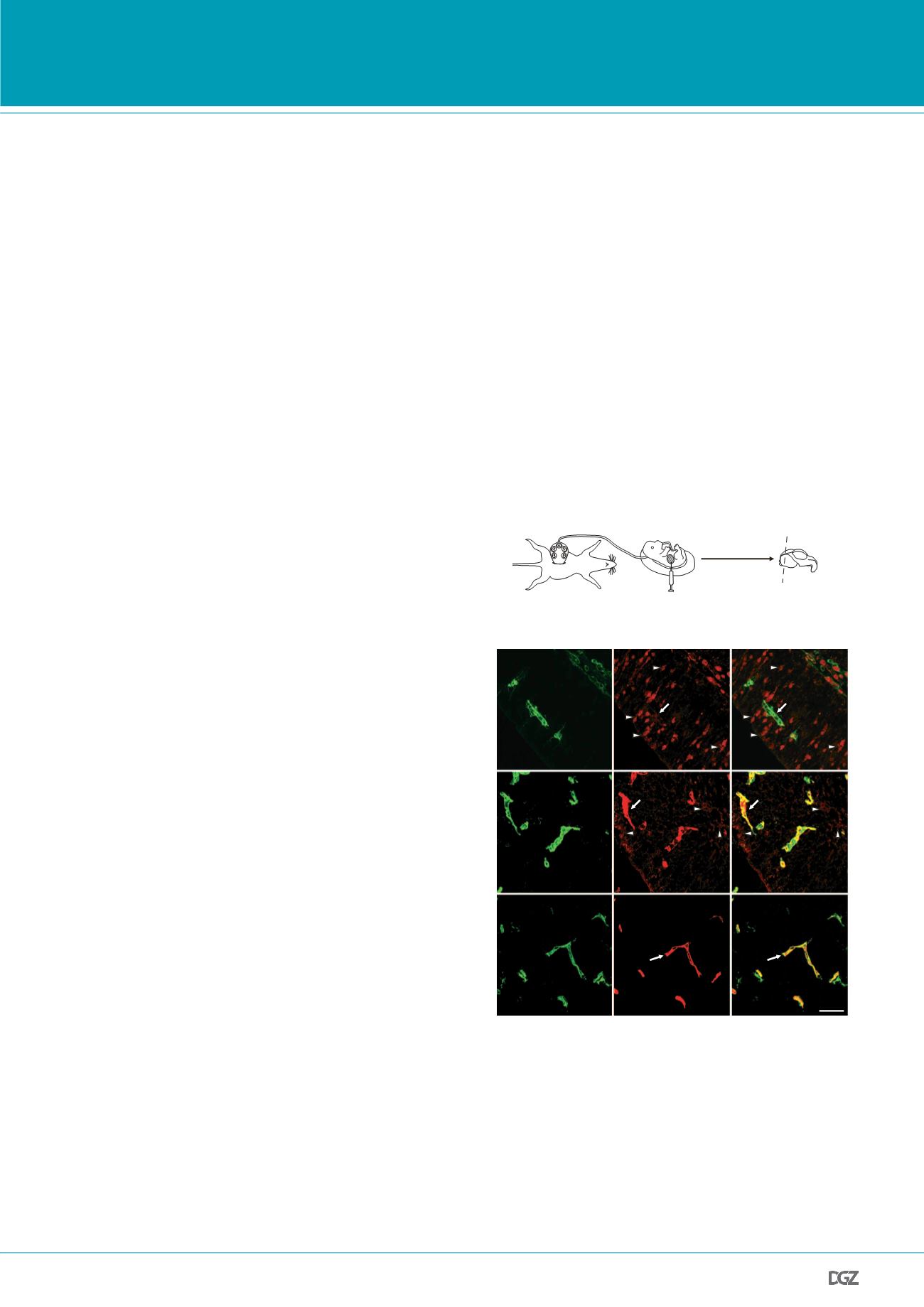
a
Step 3
Step 2
Step 1
Embryos are exposed
from anaesthetized dams
Tracer injection
into embryonic livers
Embryonic brains
are fixed and sectioned
50 µm
3 min of tracer
circulation
Figure 1
|
A novel tracer-injection method reveals a temporal profile of
functional BBB formation in the embryonic cortex. a
,
In utero
embryonic
liver tracer injection method; fenestrated liver vasculature enabled rapid tracer
uptake into the embryonic circulation.
b
, Dextran-tracer injection revealed a
temporal profile of functional cortical BBB formation. Representative images of
dorsal cortical plates from injected embryos after capillary labelling with lectin
(green, lectin; red, 10-kDa tracer). Top panel (E13.5), tracer leaked out of
capillaries and was subsequently taken up by non-vascular parenchyma cells
(arrowheads), with little tracer left inside capillaries (arrow). Middle panel
(E14.5), tracer was primarily restricted to capillaries (arrow), with diffused
tracer detectable in the parenchyma (arrowheads). Bottompanel (E15.5), tracer
was confined to capillaries (arrow).
n
5
6 embryos (3 litters per age).
2,0
4,0
6,0
8,0
10,0
12,0
14,0
16,0
Lung endothelial expression (a.u.)
a
Expression (a.u.)
1,00
2,00
3,00
4,00
5,00
6,00
7,00
b
c
2,00
4,00
6,00
8,00
10,00
12,00
Expression (a.u.)
Figure 2
|
a
, Dot-plo
transcript
isolated at
expressio
values ab
indicate a
blue.
b
, P
astrocyte,
both corte
transcript
significan
mean
6
s.
RESEARCH
LETTER
2 | N A T U R E | V O L 0 0 0 | 0 0 M O N T H 2 0 1 4
Macmillan Publishers Limited. All
©2014
Figure 1 | A novel tracer injection method reveals a temporal profile
of functional BBB formation in the embryonic cortex:
a, In-utero
embryonic liver tracer injection method - fenestrated liver vasculature
allowed rapid tracer uptake into the embryonic circulation. b, 10-kDa
dextran-tracer injection revealed a temporal profile of functional cortical
BBB formation. Representative images of dorsal cortical plates from injec-
ted embryos after capillary labeling with lectin (Green: lectin, red: 10-kDa
tracer). Upper panel, E13.5: Tracer leaked out of capillaries and was sub-
sequently taken up by non-vascular parenchyma cells (arrowheads), with
little tracer left inside capillaries (arrow). Middle panel, E14.5: Tracer was
primarily restricted to capillaries (arrow), with diffused tracer detectable in
the parenchyma (arrowheads). Lower panel, E15.5: Tracer was confined to
capillaries (arrow). n=6 embryos (3 litters/age).
PRIZE WINNERS


