9
Cell News 2/2015
PRIZE WINNERS
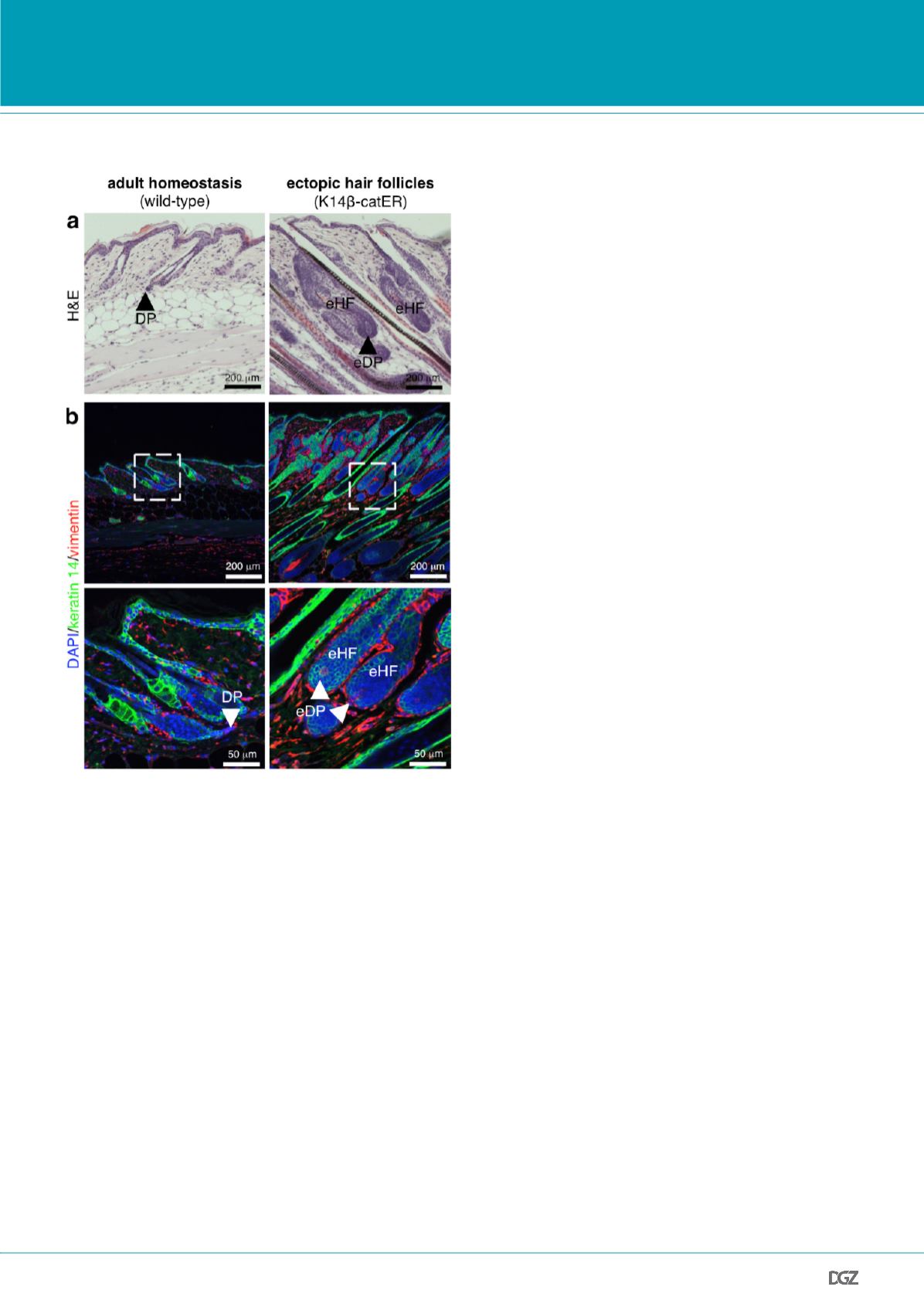
Figure 2: Sustained epidermal
β
-catenin activation induces ectopic
hair follicles with associated ectopic dermal papillae.
(Paraffin sections of back skin collected from mice positive (right panel) or
negative (left panel) for the K14
β
-catER transgene, which can be activated
by application of 4-hydroxytamoxifen (six doses of 1.5 mg 4-hydroxytam-
oxifen over two weeks).
(a) Haematoxylin and eosin (H&E) stained sections showing dermal papillae
(DPs) in wild-type skin (hair follicle resting stage: telogen; left panel) and
ectopic hair follicles (eHFs) with associated ectopic DPs (eDPs) in transge-
nic mice (right panel).
(b) Sections were labelled with antibodies against keratin 14 (green)
and vimentin (red). Nuclei were counterstained with 4',6-diamidino-
2-phenylindole (DAPI). Inserts indicate higher magnifications shown below.
Arrowheads indicate areas of DPs and ectopic DPs, respectively.
protein 1 (BLIMP1; also known as PRDM1) as a marker of sebocyte
progenitors residing in the HF adjacent to the SG (Horsley
et al.
,
2006). This study remained controversial, as BLIMP1 was shown
to be expressed by terminally differentiated cells in all epidermal
compartments, including the SG (Magnusdottir
et al.
, 2007; Cottle
et al.
, 2013). We have now re-assessed the role of BLIMP1-expres-
sing cells in the epidermis (Kretzschmar
et al.
, 2014).
First, we examined the expression of BLIMP1 in murine back skin
at different postnatal time points. BLIMP1 expression was limi-
ted to non-dividing, terminally differentiated epidermal cells
of the IFE, HF and SG, but not detectable in proliferating sebo-
cyte progenitors. Consistent with this observation in mouse skin,
BLIMP1 was expressed in terminally differentiated cells in all
epidermal compartments of human skin. In human sebaceous
tumours, BLIMP1 expression was confined to the most differentia-
ted cells. We subsequently analysed adult skin collected from mice
with epidermal-specific loss of
Blimp1
and, similarly to Horsley
et
al.
(2006), found an enlargement of the SG. However, we identified
multiple other deficiencies that were not restricted to the SG in
adult mouse skin, such as hyperplasia and perturbed differentiation
of IFE and HF infundibulum. In addition, colony-forming efficiency
assays did not demonstrate any significant increase in clonoge-
nic potential of isolated BLIMP1-positive sebocytes compared to
BLIMP1-negative sebocytes. In order to understand whether dif-
ferentiated sebocytes might originate from a subset of BLIMP1-
positive cells, as reported by Horsley
et al.
(2006), we performed
genetic lineage-tracing experiments (Kretzschmar and Watt,
2012). However, we did not find any evidence for BLIMP1-positive
cells that gave rise to proliferative and differentiating sebocytes
in
vivo
(Fig. 1). In a different set of lineage-tracing experiments we
showed that leucine-rich repeat-containing G-protein coupled re-
ceptor 6 (LGR6) and leucine-rich repeats and immunoglobulin-like
domains protein 1 (LRIG1) are expressed in those SG stem cells that
generated all sebocyte lineages, including terminally differentiated
sebocytes positive for BLIMP1 (Kretzschmar
et al.
, 2014).
In conclusion, our results demonstrate that BLIMP1 is expressed
by terminally differentiated cells and is required for postnatal
homeostasis in multiple epidermal compartments, but that it does
not define a sebocyte progenitor population (Kretzschmar
et al.
,
2014). Our research strongly suggests that the SG is maintained
in an autonomous fashion by a distinct pool of epidermal stem
cells, similarly to other epidermal compartments, such as the HF,
in agreement with recent work (Page
et al.
, 2013; Kretzschmar and
Watt, 2014).
The dermal niche of epidermal stem cells is an unexpec-
tedly plastic tissue
During skin development, HF morphogenesis is dependent on epi-
dermal activation of Wnt/
β
-catenin signalling (Watt and Collins,
2008). Whereas new HFs do not form during adult homeostasis,
HF-like structures can develop from all epidermal compartments
in response to oncogenic or wound-induced epidermal
β
-catenin
activation. In adult skin, sustained epidermal
β
-catenin activation


