11
Cell News 2/2015
PRIZE WINNERS
While dermal areas adjacent to the sites of ectopic HF formati-
on were comprehensively remodelled, distal areas did not show
significant fibroblast proliferation or changes in the extracellular
matrix composition. This observation led us to investigate whether
the differential dermal responses to epidermal signalling might be
the result of marked dermal heterogeneity. Using genetic lineage
tracing and skin reconstitution assays, we were able to show that
indeed two distinct lineages of dermal fibroblasts are established
during embryonic skin development (Driskell
et al.
, 2013). In the
upper dermal lineage, dermal fibroblasts generated the papillary
dermis containing the DP and the arrector pili muscle, which con-
trols piloerection (Fujiwara
et al.
, 2011). Conversely, fibroblasts of
the lower dermal lineage formed the reticular dermis and the hy-
podermal pre-adipocytes and adipocytes. Our study indicated that
in the first stages of skin wound healing, fibroblasts of the lower
dermis expanded (Driskell
et al.
, 2013). Later, during IFE repair, fib-
roblasts of the papillary dermis were stimulated. In addition, sus-
tained activation of
β
-catenin in the epidermis using K14
β
-catER
transgenic mice provided strong extrinsic cues to the upper dermal
lineage, which led to
de novo
HF formation after wounding in adult
skin. Taken together, our results revealed the cellular origins of der-
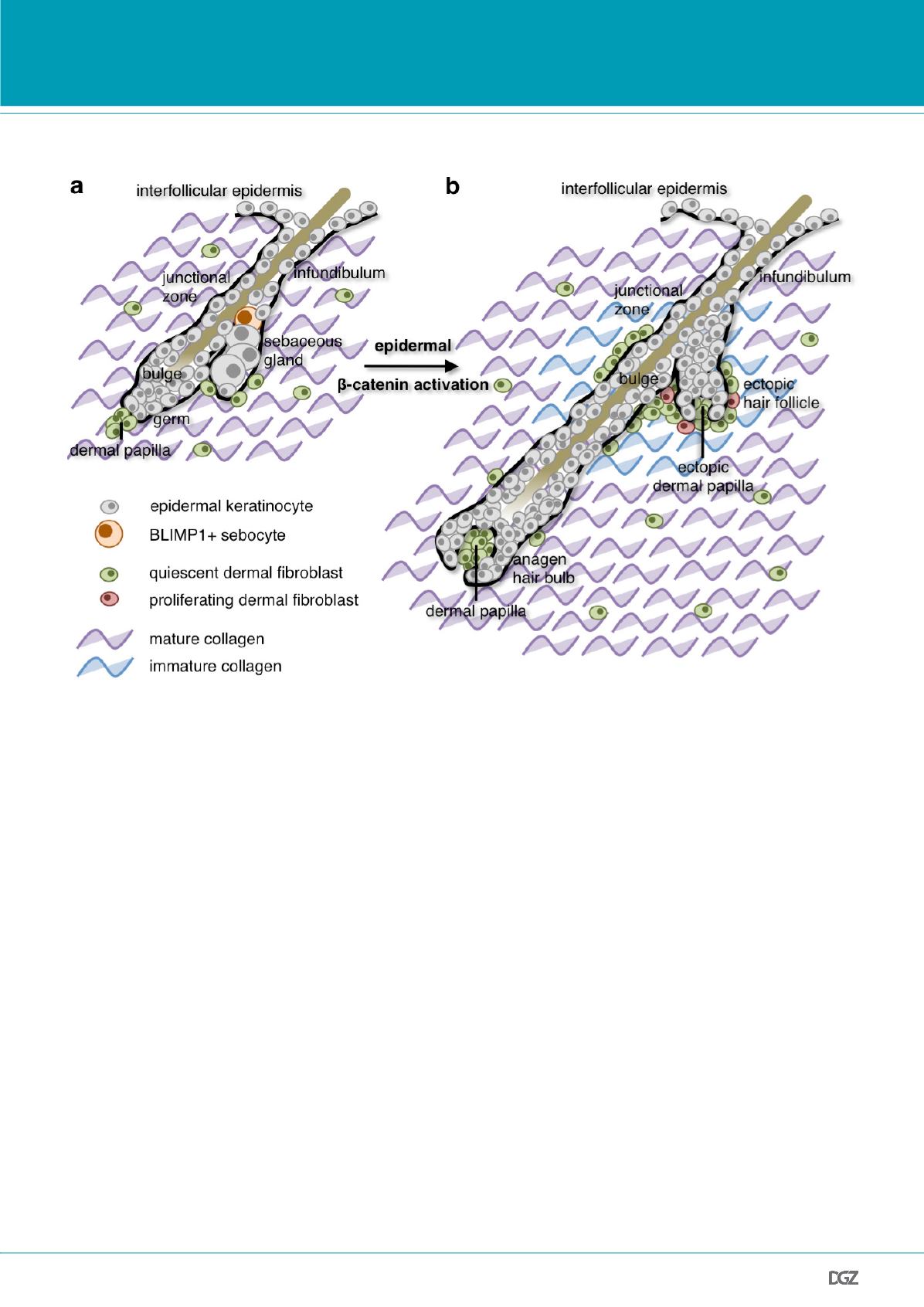
Figure 4: Summary of the results presented in this article.
(a) In mouse skin, BLIMP1+ cells in the sebaceous gland (orange) are terminally differentiated sebocytes (Kretzschmar et al., 2014). Also, adult dermis is
rich in highly crosslinked, mature collagen and has a low fibroblast density in wild-type skin (Collins
et al.
, 2011).
(b) Sustained epidermal
β
-catenin activation induces hair follicle growth (anagen) and triggers transformation of sebaceous glands into ectopic hair
follicles. At sites of ectopic hair follicle formation, dermal fibroblasts are reprogrammed to proliferate and produce immature collagen (Collins
et al.
,
2011). This comprehensive remodelling resembles the dermis found in neonatal skin.
mal fibroblast heterogeneity providing new insights into the me-
chanisms of wound repair and HF neogenesis in adult skin (Driskell
et al.
, 2013).
In conclusion, our studies strongly suggest that while dermal hete-
rogeneity is established during early skin development (Driskell
et
al.
, 2013), the postnatal dermis remains plastic and can be compre-
hensively remodelled in response to extrinsic cues from the epider-
mal stem cell compartment (Fig. 4) (Collins
et al.
, 2011).
Conclusions and outlook
The results presented here provide new insights into the biology of
adult mammalian skin. Both, epidermis and dermis, become highly
compartmentalised during early skin development, yet remain un-
expectedly plastic tissues in the adult (Blanpain and Fuchs, 2014;
Donati and Watt, 2015; Driskell and Watt, 2015). Reciprocal signal-
ling between the two skin layers are crucial for skin morphogenesis
and homeostasis (Solanas and Benitah, 2013; Hsu
et al.
, 2014). Yet,
our studies, which have unravelled a capacity of epidermal stem
cells to comprehensively remodel their dermal niche, will have ma-
jor implications for futures studies on skin regeneration and disea-
ses such as cancer.


