21
Cell News 2/2015
vessels in
Mfsd2a
-\-
embryonic brains and was found in the cor-
tical parenchyma (Fig. 3a) and individual parenchyma cells (Fig.
3b). Furthermore, using imaging and spectrophotometric quan-
tification methods, we found that the leaky phenotype persisted
in early postnatal and adult (Fig. 3c)
Mfsd2a
-\-
mice. This leaky
phenotype was consistent for a large range of tracers of different
molecular weights and different molecular compositions (Sulfo-
NHS-biotin (~550 Da), horseradish peroxidase (HRP; ~44 kDa)
and 70-kDa dextran). In contrast to severe barrier leakage defects,
brain vascular patterning was similar between
Mfsd2a
-\-
mice and
littermate controls (No abnormalities were identified in capillary
density, capillary diameter, vascular branching or in cortical ar-
terial distribution). Therefore, MSFD2A is specifically required for
proper formation of a functional BBB but not for CNS vascular
morphogenesis
in vivo
.
We where particularly intrigued by the cell biological property of
BBB endothelial cells, effected by the genetic ablation of
Mfs-
d2a
, that mediates the leaky BBB phenotype. Electron microscopy
examination reveals a dramatic increase in CNS-endothelial-cell
vesicular transcytosis in
Mfsd2a
-/-
mice, without obvious tight-
junction defects. We examined these properties by electron mi-
croscopy in embryonic brains and P90 mice following intravenous
HRP injection
3
. Electron microscopy failed to reveal any apparent
abnormalities in the ultrastructure of endothelial tight junctions
(Fig. 5a). In electron micrographs of cerebral cortex in HRP-injec-
ted adults, peroxidase activity was revealed by an electron-dense
reaction product that filled the vessel lumen. In both control and
Mfsd2a
-/-
mice, HRP penetrated the intercellular spaces between
neighboring endothelial cells only for short distances. HRP was
stopped at the tight junction, creating a boundary between HRP-
positive and HRP-negative regions without leakage through tight
junctions (Fig. 4a). In contrast, CNS endothelium of
Mfsd2a
-/-
mice
displayed a dramatic increase in the number of vesicles, including
luminal and abluminal plasma membrane-connected vesicles and
free cytoplasmic vesicles, which may indicate an increased rate
of transcytosis (Fig. 4b). Specifically, pinocytotic events were evi-
denced by type II lumen-connected vesicles pinching from the lu-
minal plasma membrane. Greater than twofold increases in vesic-
le number in
Mfsd2a
-/-
mice compared to control littermates were
observed in different locations along the transcytotic pathway
(Fig. 4). Furthermore, the HRP reaction product in adult mice was
observed in vesicles invaginated from the luminal membrane and
exocytosed at the abluminal plasma membrane only in
Mfsd2a
-/-
mice (Fig. 4d), suggesting that HRP was subject to transcytosis in
these animals but not in wild-type littermates.
In addition to the suggested
MFSD2A
function at the BBB we
found indications for its cellular localization and for regulation
of its expression. We found evidence indicating that that
Mfs-
d2a
endothelial expression is regulated by pericytes to facilitate
BBB integrity. By immuno-electron-microscopy examination, we
found
MFSD2A
protein in the luminal plasma membrane and as-
sociated with vesicular structu-
res in cerebral endothelial cells,
but not in tight junctions. At pre-
sent, it is not clear whether the
reported transporter function of
MFSD2A
is related to its role in
BBB formation.
BBB breakdown has been repor-
ted in the etiology of various neu-
rological disorders
2
, and two se-
parate
Mfsd2a
-deficient mouse
lines were reported to exhibit
neurological abnormalities, such
as ataxic behaviour
15,18
. Finding a
novel physiological role of
MFS-
D2A
may provide a valuable tool
to address how a non-functional
BBB could affect brain develop-
ment. In addition, our finding
also highlights the importance
of the transcytotic mechanism in
BBB function, whereas most pre-
vious attention has been focused
on potential BBB leaks through
intercellular junctions. Indeed,
increased numbers of pinocytotic
vesicles were observed following
acute exposure to external stress
inducers in animal models
19
, and
d from the luminal membr ne and exocytos d at the ablum-
a memb ane o ly in
Mfsd2a
2
/
2
mice (Fig. 5d), sugg sting
was subject to transcytosis in these animals but not in wild-
ates (ExtendedDataTable 2). Together, these findings suggest
that the BBB leakiness observed in
Mfsd2a
2
/
2
mice was
opening of tight junctions, but rather by increased tran
ficking across the endothelial cytoplasm.
Studies using pericyte-deficient genetic mouse model
that pericytes can also regulate BBB integrity. Thesemice
vesicle trafficking without obvious junction defects
4,5
, sim
servations in
Mfsd2a
2
/
2
mice. We therefore examined th
that Mfsd2a may regulate CNS endothelial transcytosis b
pericyte funct on or hat the ffect of p ricyt s on endot
tosis is mediated by Mfsd2a. First, pericyte coverage, atta
capillary wall, and pericyte ultrastructure and positioni
endothelial cells were normal in
Mfsd2a
2
/
2
mice (Extende
These data, t geth r with the lack of Mfsd2 expressio
suggest that th increased transcy osis observed in
Mfsd2a
lial cells is not secondary to pericyte abnormalities. Sec
reduction in pericyte coverage can influence endothelial ge
2a
P2
f
Pdgfr
β
**
*
P5
Lectin
Mfsd2a
Mfsd2a/Claudin-5/DAPI
PECAM
PECAM
sd2a
Mfsd2a/Claudin-5
Claudin-5
Mfsd2a/
Claudin-5
Mfsd2a
Mfsd2a/
Pdgfr
β
E15.5
Mfsd2a
100 µm
100 µm
100 µm
5 µm
100 µm
Mfsd2a +/+
Mfsd2a +/+
a
10-kDa tracer
Lectin
Overlay
b
E15.5
Percentage of sample
0
100
80
60
40
20
0
0
0.5
1
1.5
2
2.5
Spectrophotometric values
(fold change)
WT MUT
P90
*
c
d
E15.5
Mfsd2a +/+
Mf d2a –/–
50 μm
0
20
40
60
80
100
Vascular density
(% of WT)
120
WT MUT
0
20
40
60
80
100
120
Number of branch
points (% of WT)
WT MUT
Capillary mean
diameter (μm)
N.S.
0
5
10
15
20
25
WT
MUT
25 μm
Mfsd2a +/+
Mfsd2a –/–
Mfsd2a –/–
Mfsd2a –/–
Mfsd2a –/–
25 μm
25 μm
Tracer-filled parenchyma cells per section
10-40
5-10
1-5
Mfsd2a
is required for the establishment of a functional BBB but
S vascular patterning
in vivo
. a
,
b
, Dextran-tracer (10 kDa)
t E15.5 revealed a defective BBB in mice lacking
Mfsd2a
.
a
, The
confined to the capillaries (arrow) in wild-type littermates, whereas
embryos showed large amounts of tracer leakage in the brain
a (arrowheads).
b
, Capillaries (arrows) surrounded by tracer-filled
chyma cells (arrowheads) in
Mfsd2a
2
/
2
cortex. Quantification of
parenchyma cells in control versus
Mfsd2a
2
/
2
cortical plates
nel,
n
5
7 embryos per genotype).
c
, Spectrophotometric
quantification of 10-kDa dextran-tracer from cortical extracts of
post intravenous injection, indicating that BBB leakiness in
Mf
persists into adulthood (
n
5
3 mice per genotype).
d
,
Mfsd2a
2
exhibit normal vascular patterning. No abnormalities were fou
vascular density, branching and capillary diameter (E15.5; gree
Quantification of wild-type and
Mfsd2a
2
/
2
samples (
n
5
4 em
genotype). All data are mean
6
s.e.m. MUT, mutant; N.S., not s
wild type.
*
P
,
0.05 (Mann–Whitney
U
-test).
in BBB-containing CNS vasculature but not in vasculature of t
plexus (left, dashed line), or outer meninges or skin (right, red
e–g
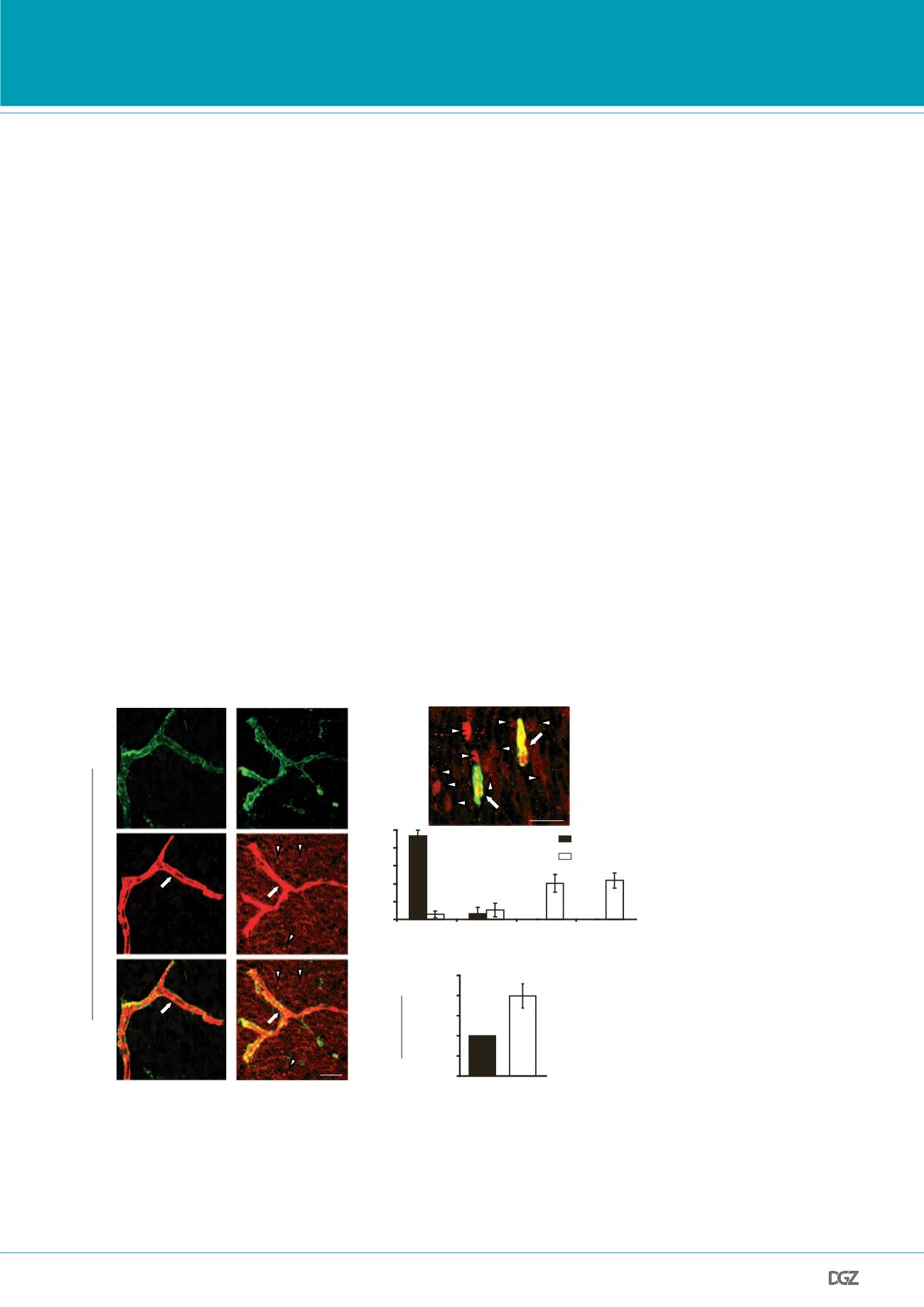
, Immunohistochemical staining of Mfsd2a protein shows s
expression in CNS endothelial cells (red, Mfsd2a; green, claudi
(endothelium); blue, DAPI (nuclei); grey, Pdgfr
b
(pericytes)).
expression in the brain vasculature of wild-type mice (top pan
Mfsd2a
2
/
2
mice (bottom panel).
f
, Mfsd2a expression only in
positive endothelial cells (arrow; endothelial nucleus is indicated
but not in adjacent pericytes (arrowhead; pericyte nucleus is in
double asterisk).
g
, Lack of Mfsd2a expression in choroid plex
(fourth ventricle coronal view, dashed line), as opposed to the
Mfsd2a expression in cerebellar vasculature.
n
5
3 embryos (3
Figure 3 |
Mfsd2a
is required for the establishment of a functional BBB but not for CNS vascular patter-
ning
in vivo
:
a,b, 10-kDa dextran-tracer injections at E15.5 revealed a defective BBB in mice lacking
Mfsd2a
. a,
The tr cer was confi ed to the capillaries (ar ow) in wild-type littermates, whereas
Mfsd2a
-/- embryos how d
large amounts of tracer leakage in the brain parenchyma (arrowheads). b, Capillaries (arrows) surrounded by
tracer-filled brain parenchyma cells (arrowheads) in
Mfsd2a
-/- cortex. Quantification of tracer-filled parenchy-
ma cells in control versus
Mfsd2a
-/- cortical plates (lower panel n=7 embryos/genotype). c, Spectrophotometric
quantification of 10-kDa dextran-tracer from cortical extracts, 16hr post i.v. injection, indicating that BBB
leakiness in
Mfsd2a
-/- mice persists into adulthood (n=3 mice/genotype). All data are mean±s.e.m.
PRIZE WINNERS


