15
Cell News 2/2015
on of erroneous microtubule-kinetochore attachments during
the early phases of mitosis. Such faulty attachments are cha-
racterized by kinetochores that are concomitantly attached to
microtubules emanating from the two opposing poles (so-called
merotelic attachments). This can also occur during a normal mi-
tosis by chance, but is usually corrected before anaphase onset.
If the rate of generation of merotelic kinetochore attachments
exceeds the rate of correction so-called lagging chromosomes
appear during anaphase. They reflect single chromatids which
harbor a kinetochore that is concomitantly bound to microtu-
bules from both poles (Fig. 1). As a consequence, such chro-
matids cannot properly be segregated onto a pre-determined
daughter cell and thus, repre-
sent a pre-stage of chromoso-
me missegregation (Gregan
et
al.
, 2011). Importantly, lagging
chromosomes are frequently
detected specifically in cancer
cells exhibiting CIN indicating
that erroneous and abnormally
stable microtubule-kinetocho-
re attachments that are not
properly corrected represent a
major source for chromosome
missegregation in human can-
cer cells.
In addition to supernumerary
centrosomes also other me-
chanisms might contribute to
the generation of microtubule-
kinetochore mal-attachments
(Nam
et al.
, 2015). For in-
stance, timely separation and
proper positioning of the two
centrosomes as well as correct
assembly and composition of
kinetochores are crucial for
proper interaction with micro-
tubules. Thus, various defects
can contribute to the genera-
tion of erroneous microtubule-
kinetochore attachments, but
whether they are indeed pre-
sent in cancer cells remains to
be shown.
Chromosomally instable
cancer cells exhibit incre-
ased microtubule dynamics
It is conceivable that highly
dynamic microtubules are pi-
votal for the faithful executi-
on of mitosis. In particular, the
plus ends of microtubules ex-
hibit ongoing transitions from
a growing to a shrinking state
(called "catastrophe") and
vice versa
(called "rescue"), which al-
lows the "search and capture" of kinetochores in order to achie-
ve efficient chromosome alignment (Kline-Smith and Walczak,
2004). Thus, abnormalities in microtubule dynamics are expec-
ted to result in impaired chromosome alignment and might re-
present a conceivable cause for CIN in cancer cells. Therefore,
we focused on potential abnormalities in microtubule plus end
dynamics in human cancer cells as a source for CIN (Ertych
et al.
,
2014). For our analyses we chose colorectal cancer (CRC) cells,
since this tumor entity is a prime example for a tumor exhibiting
CIN. In fact, about 85% of CRC cases are characterized by CIN
and high-grade aneuploidy while the remaining cases maintain
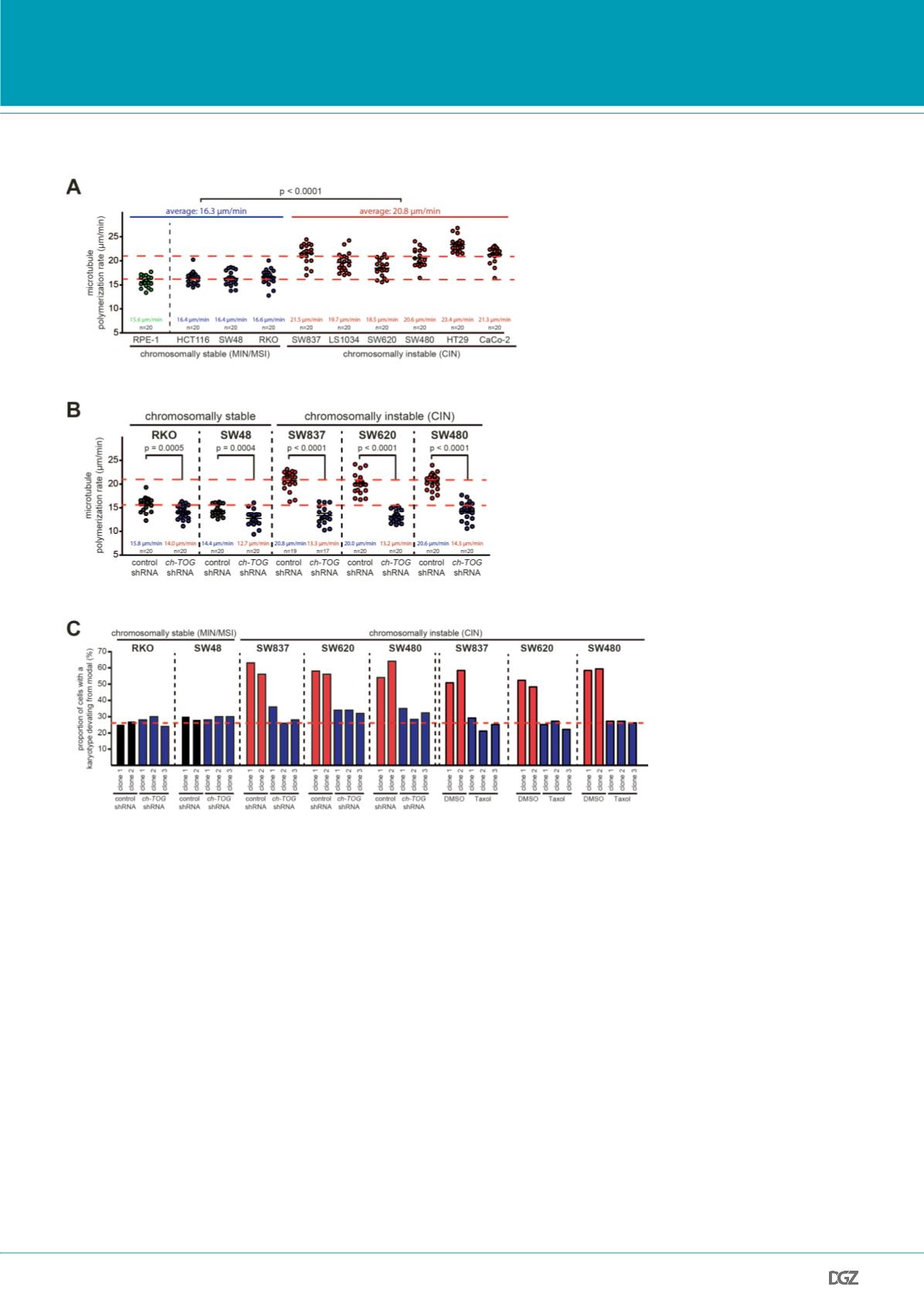
Figure 2:
Increased microtubule assembly rates trigger CIN. (A) CIN cells exhibit higher microtubule plus end
assembly rates. Measurement of microtubule plus end assembly rates was performed in various human colon
cancer cell lines exhibiting MIN/MSI or CIN by tracking EB3-GFP for 20 individual microtubules per cell (n=20;
t-test). (B) Restoration of normal microtubule assembly rates in CIN cells by partial repression of ch-TOG/CKAP5.
The indicated MIN/MSI or CIN cell lines were stably transfected with shRNAs targeting ch-TOG/CKAP5 and micro-
tubule assembly rates were determined. (C) Restoration of normal microtubule assembly rates suppresses CIN in
otherwise chromosomally instable cancer cells. Single cell clones were generated for the indicated cell lines and
numerical karyotype variability that evolved over 30 generations was determined as a measure for CIN. For each
clone the numerical chromosome composition for at last 100 metaphase cells was evaluated.
From: Ertych
et al.
, 2014.
PRIZE WINNERS


