22
Cell News 2/2015
have also been observed in human pathological conditions
20
. It
will be interesting to examine whether
MFSD2A
is involved in
these pathological and acute assault situations. Our identification
of a key molecular player in BBB formation may also aid efforts to
develop therapeutic approaches for efficient drug delivery to the
CNS. As an accessible cell surface molecule,
MFSD2A
is poised to
be a potential therapeutic target for BBB restoration and mani-
pulation.
Acknowledgements
I wish to thank Dr. Chenghua Gu for her mentorship and support
throughout my postdoctoral research at her lab and for her cons-
tant support and guidance in my scientific carrier.
I wish to thank Baptiste Lacoste, Esther Kur, Benjamin J. Andre-
one, Yoav Mayshar and Han Yan
for their important contribution
to this work. Special thanks to all
past and present members of the
Gu lab.
Finally I wish to thank M. Kar-
novsky, E. Raviola and M. Green-
berg for advice and for valuable
scientific discussion.
Many thanks for the Werner
Risau-Prize committee and the
DGZ for recognizing our work. I
feel grateful for this honor, espe-
cially being part of the growing
community of developmental va-
scular biology and BBB, research
topics which Werner Risau pio-
neered.
References
1. Saunders, N.R., Liddelow, S.A. & Dziegielews-
ka, K.M. Barrier mechanisms in the developing
brain. Front Pharmacol. 3, 46 (2012).
2. Zlokovic, B. V. The blood-brain barrier in
health and chronic neurodegenerative disor-
ders. Neuron 57, 178–201 (2008).
3. Reese T.S. & Karnovsky M.J. Fine structural
localization of a blood-brain barrier to exoge-
nous peroxidase. J Cell Biol. 34, 207-17 (1967).
4. Saunders, N.R.
et al.
Transporters of the
blood–brain and blood–CSF interfaces in deve-
lopment and in the adult. Mol Aspects Med. 34,
742-752 (2013).
5. Stenman, J. M.
et al.
Canonical Wnt signa-
ling regulates organ-specific assembly and dif-
ferentiation of CNS vasculature. Science 322,
1247–1250 (2008).
6. Liebner, S.
et al.
Wnt/beta-catenin signaling
controls development of the blood-brain barri-
er. J. Cell Biol. 183, 409–417 (2008).
7. Daneman, R.
et al.
Wnt/b-catenin signaling
is required for CNS, but not non-CNS, angioge-
nesis. Proc. Natl Acad. Sci. USA. 106, 641–646
(2009).
8. Tam, S.J.
et al.
Death receptors DR6 and TROY
regulate brain vascular development. Dev Cell.
22, 403-17 (2012).
9. Cullen, M.
et al.
GPR124, an orphan G
protein-coupled receptor, is required for CNS-
specific vascularization and establishment of
the blood-brain barrier. Proc Natl Acad Sci U S
A. 108, 5759-6 (2011).
10. Wang, Y.
et al.
Norrin/Frizzled4 Signaling in
retinal vascular development and blood brain
barrier plasticity. Cell 151, 1332-44 (2012).
11. Alvarez, J.I.
et al.
The Hedgehog pathway
promotes blood-brain barrier integrity and CNS
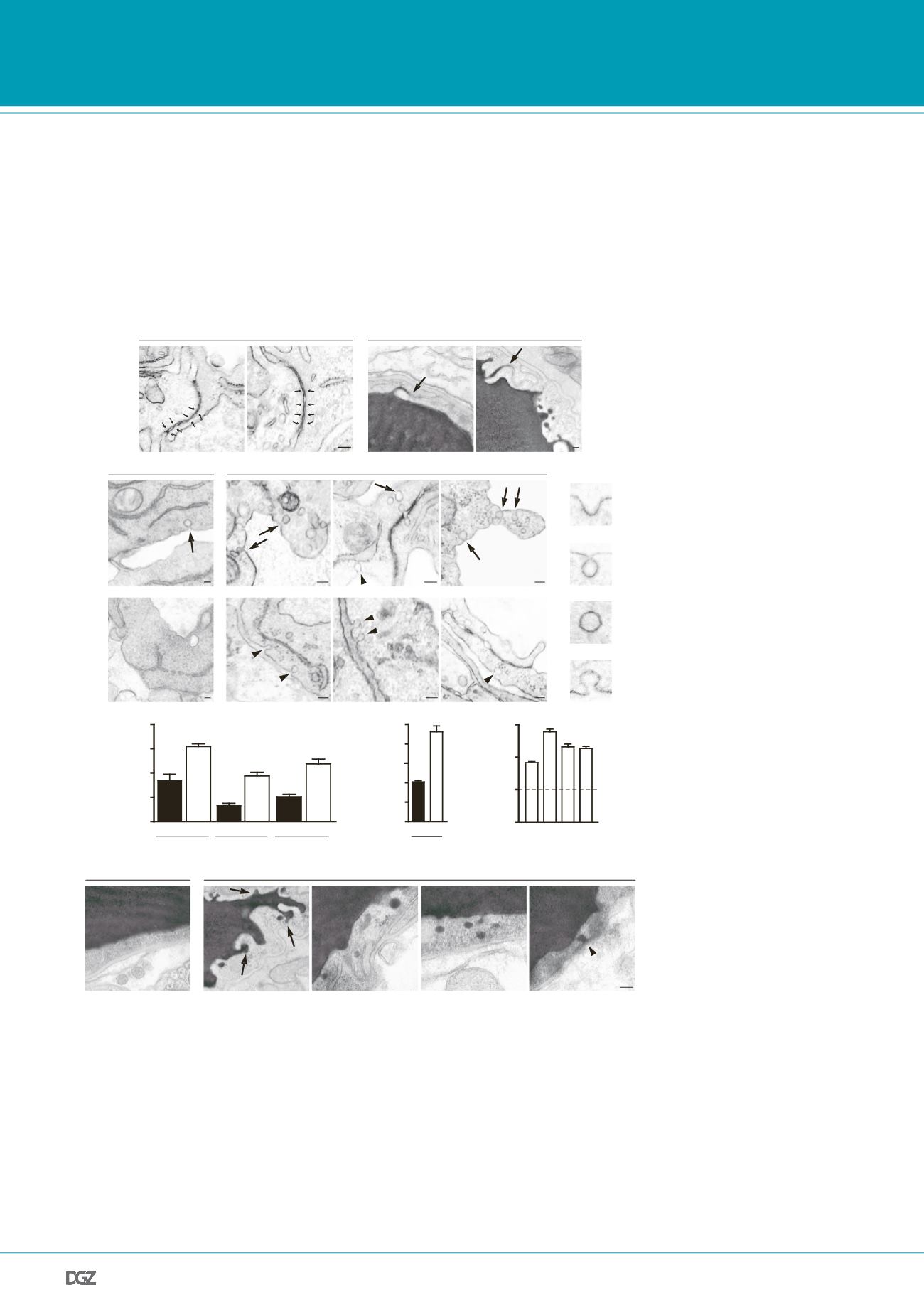
erefore we analysed published microarray data of two
ent mouse models
5
and found a dramatic downregulation
ese mice, with a direct correlation between the reduction
expression and the degree of pericyte coverage (Extended
urthermore, immunostaining for Mfsd2a in
Pdgfb
ret/ret
immuno-electron-microscopy examination, Mfsd2a proteinwas found
in the luminal plasma membrane and associated with vesicular struc-
tures in cerebral endothelial cells, but not in tight junctions (Extended
Data Fig. 8). At present, it is not clear whether the reported transporter
function of Mfsd2a is related to its role in BBB formation.
**
***
*** ***
***
**
***
***
Mfsd2a
+/+
Mfsd2a
+/+
Mfsd2a
+/+
Mfsd2a
+/+
E17.5
a
P90
L
*
L
*
b
Abluminal vesicles
Luminal vesicles
L
L
L
L
L
L
Ab
L
Ab *
Ab
L
Ab
*
E
E
E
E
E
E
E
E
E
Lum. type I
Lum. type II
Cytoplasmic
Abluminal
d
Tracer-filled
invaginations
Tracer uptake
Transcytosis
Abluminal tracer
release
L
L
L
L
L
*
*
*
*
*
E
E
E
E
E
Lum. type I
Lum. type II
Abluminal
Cytoplasmic
0
100
200
300
Mean vesicular density
(vesicles per µm
2
)
Cytoplasmic
WT MUT
Percentage of WT
0
1
2
3
4
5
0.0
0.2
0.4
0.6
0.8
Mean vesicular density
(vesicles per µm)
Lum. type I
WT MUT
Lum. type II
WT MUT
Abluminal
WT MUT
c
Mfsd2a
–/–
Mfsd2a
–/–
Mfsd2a
–/–
Mfsd2a
–/–
2a
is required specifically to suppress transcytosis in brain
maintain BBB integrity.
Electron-microscopy examination
.
a
, Embryonic
Mfsd2a
2
/
2
endothelium (E) showed no overt
ltrastructural defect (left, normal ‘kissing points’, small arrows).
n (L) in HRP-injected adult mice was filled with electron-dense
nzidine (DAB) reaction (black) that diffused into intercellular
d sharply at the junction without parenchymal leakage (right,
eased vesicular activity in embryonic
Mfsd2a
2
/
2
endothelium
ld-type endothelium displayed very few vesicles (arrow).
/
2
endothelium contained many vesicles of various types:
luminal (arrows) and abluminal (Ab; arrowheads) membrane-connected
and cytoplasmic vesicles.
c
, Vesicular density quantification (as shown in
b
, reference WT values (dashed line), see also Supplementary Fig. 7a).
d
, Increased transcytosis was evident in HRP-injected adult
Mfsd2a
2
/
2
mice
(P90). In wild-type littermates (left) HRP activity was confined to the lumen
with no HRP-filled vesicles. Many HRP-filled vesicles found in
Mfsd2a
2
/
2
endothelial cells (right, see quantification in Supplementary Fig. 7b). Luminal
invaginations (dye uptake, arrows) and release to the basement membra e
(abluminal side, asterisk). Scale bars, 100 nm (
a
,
b
), 200 nm (
c
). All data
are mean
6
s.e.m.
**
P
,
0.01,
***
P
,
0.001 (student’s
t
-test).
LETTER
Figure 4 |.
Mfsd2a
is required specifically to suppress transcytosis in brain endothelium to maintain BBB
integrity:
Electron-microscopic examination of BBB integrity. a, Embryoni
Mf d2a
-/- endothelium showed
o overt tight-juncti n ultrastructural defect (n rmal “kissing points”, small arrows, left). HRP-inject d adult
mic show d lumen filled electr n-den e DAB eaction (black) that diffused into intercellular clefts but stopped
sharply at the junction without parenchymal leakage (arrows, right). b, Increased vesicular activity in embry-
onic
Mfsd2a
-/- endothelium (E17.5). Left, wild-type endothelium displayed very few vesicles (arrow). Right,
Mfsd2a
-/- endothelium contained many vesicles of various types: luminal-(arrows), abluminal-(arrowheads)
membrane-connected and cytoplasmic vesicles. c, Vesicular density quantification (as illustrated in b, see also
Fig.S7a). d, Increased transcytosis was evident in HRP-injected adult
Mfsd2a
-/- mice (P90). In wild-type litter-
mates (left) HRP activity was confined to the lumen with no HRP-filled vesicles. Many HRP-filled vesicles found
in
Mfsd2a
/- endothelial cells (right, see quantification Fig.S7b). Luminal invaginations (dye uptake, arrows) and
release to the basement membrane (abluminal side (*). Ab: abluminal, E: endothelium, L: lumen. Scale bars: a,b:
100 nm; c, 200 nm. All data are mean±s.e.m.
PRIZE WINNERS


