14
Cell News 2/2015
Routes to CIN
In principle, any abnormality
during mitosis could affect
faithful chromosome segrega-
tion and might contribute to
CIN in cancer cells. However,
since cancer cells missegrega-
te chromosomes at rather low
rate (0.2 - 1 missegregation
events per mitosis) the under-
lying defects are expected to
be rather subtle, which make
their identification difficult
(Thompson
et al.
, 2010).
One example for a mechanism
that can cause CIN is a wea-
kened spindle assembly check-
point (SAC). This mitotic sur-
veillance pathway ensures that
every mitotic chromosome is
properly attached to spindle
microtubules and fully aligned
on the metaphase plate befo-
re sister chromatid separation
is initiated (Lara-Gonzalez
et
al.
, 2012). Complete loss of
the SAC is lethal, but parti-
al loss of checkpoint signa-
ling results in premature loss
of sister chromatid cohesion
in the presence of unaligned
chromosomes, which results
inevitably in missegregation.
Impairment of the SAC can
be achieved experimentally
by decreasing the expression
of various spindle checkpoint
genes including
MAD1, MAD2,
BUB1, BUBR1
and
MPS1
, both
in tissue culture cells and in
mouse models. In particular,
the SAC mouse models have
been very valuable to demonstrate that the induction of CIN
and aneuploidy can contribute to tumorigenesis and tumor
progression (Schvartzman
et al.
, 2010). However, checkpoint
impairment is rare in human cancer, which might reflect the
essential nature of this checkpoint surveillance mechanism. In-
terestingly, premature loss of sister chromatid cohesion during
mitosis can be mediated also by other means, e.g. upon loss
of components of the cohesion complex, a situation, which is
indeed detectable in cancer cells, albeit again at rather low fre-
quencies (Thompson
et al.
, 2010).
An important abnormality, which is frequently seen in human
cancer cells, however, is the presence of supernumerary cen-
trosomes, which result either from cytokinesis defects or from
their uncontrolled amplification during interphase (Anderhub
et
al.
, 2012). It was assumed for a long time that supernumera-
ry centrosomes give rise to the formation of multipolar mitotic
spindles, which, in turn, lead to missegregation of chromoso-
mes. However, recent detailed studies have revealed that the
formation of multipolar spindles is inevitably associated with
cell death rather than with the generation of aneuploid progeni-
tors. To evade this fate, cancer cells cluster their supernumerary
centrosomes at two opposing poles, thereby allowing the for-
mation of a pseudo-bipolar spindle that is capable to segregate
chromosomes (Ganem
et al.
, 2009). Intriguingly, the formation
of a pseudo-bipolar spindle is often preceded by a transient
multipolar spindle intermediate, which facilitates the formati-
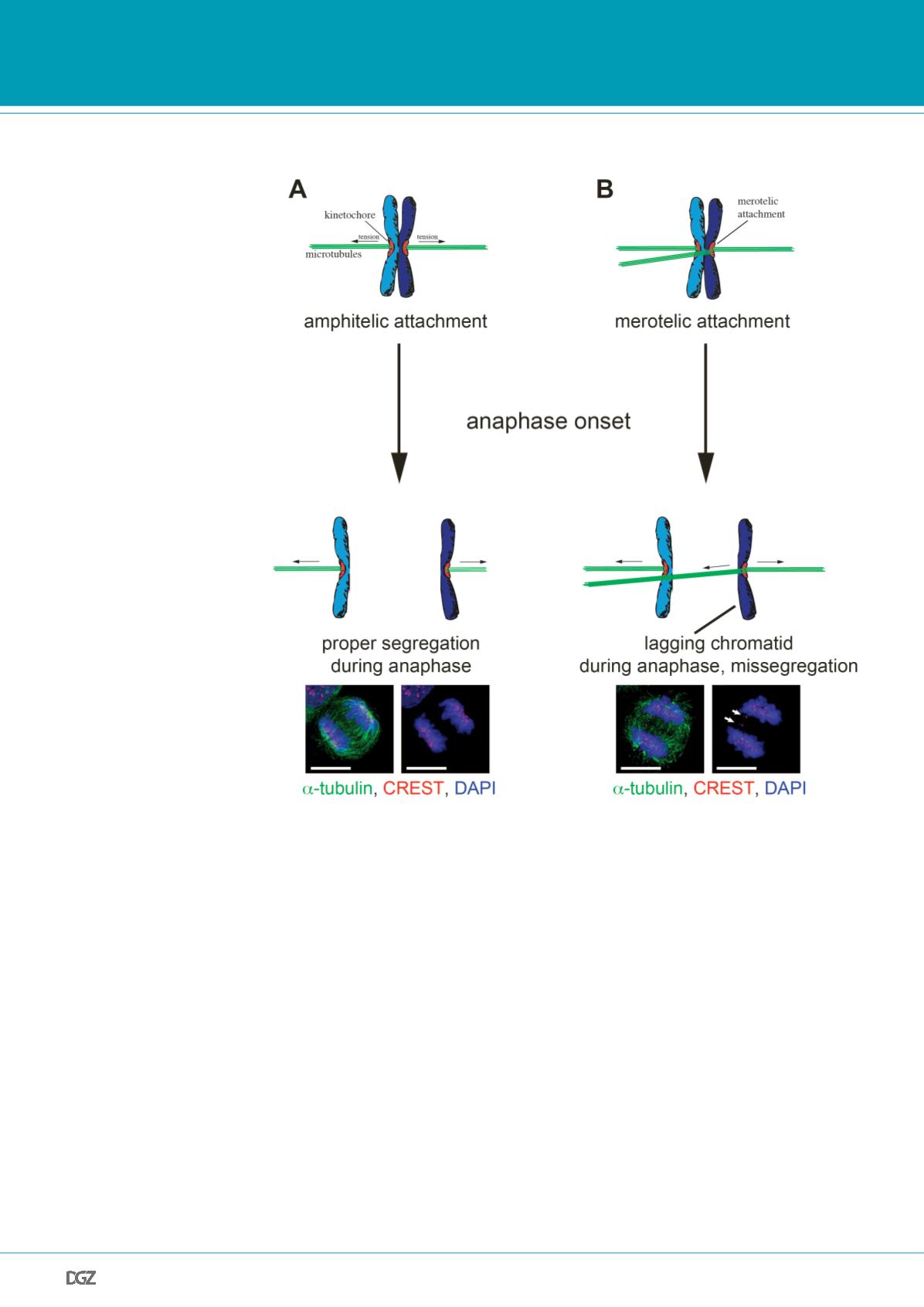
Figure 1:
Erroneous microtubule-kinetochore attachments cause chromosome missegregation. (A) Faithful
chromosome segregation requires amphitelic attachments of all chromosomes to spindle microtubules. In this
case, the two sister kinetochores are attaches to microtubules emanating from the two opposing centrosomes,
respectively. (B) Kinetochore attachments that involve concomitant binding of microtubules emanating from the
two opposing poles are referred to as merotelic attachments. If not resolved, such erroneous attachments cause
lagging chromosomes during anaphase, which cannot be properly segregated. Examples of anaphases in human
colon carcinoma cells with or without kinetochore-positive lagging chromosomes are given (scale bar, 10 µm).
PRIZE WINNERS


