8
Cell News 2/2015
PRIZE WINNERS
NIKON YOUNG SCIENTIST AWARD 2015
Remodelling adult skin by epidermal
β
-catenin activation
Kai Kretzschmar
The epidermis, the uppermost layer of mammalian skin, is compart-
mentalised into a stratified epithelium, the interfollicular epidermis
(IFE), and associated hair follicles (HFs), sebaceous glands (SGs) and
sweat glands. Adult stem cells residing in the basal layer of the
epidermis maintain tissue homeostasis and contribute to regene-
ration upon tissue damage. Multiple stem cell subpopulations have
been identified in murine epidermis in recent years (Kretzschmar
and Watt, 2014). Epidermal stem cells are in close contact with
their mesenchymal niche, the collagen-rich dermis, which provides
critical signals that regulate skin development and homeostasis
(Hsu
et al.
, 2014). This dermal-epidermal communication is media-
ted by specialised dermal compartments such as the dermal papilla
(DP), which is located at the HF base and orchestrates HF growth
throughout adult life (Driskell
et al.
, 2011). Mechanisms controlling
adult stem cell behaviour are frequently perturbed in pathological
conditions such as baldness, scar formation or skin cancer (Arwert
et al.
, 2012); yet they remain poorly understood. We therefore
wanted to investigate the intrinsic and extrinsic cues regulating
plasticity of epidermal stem cells and their dermal niche in adult
murine skin, to gain new mechanistic insights that could also have
implications in human disease.
BLIMP1 is required for postnatal epidermal homeostasis,
but is not a marker of sebocyte progenitors
Historically, epidermal homeostasis has been attributed to a single
population of epidermal stem cells that resides in a compartment
in the lower HF, the so-called bulge (Cotsarelis
et al.
, 1990). How-
ever, multiple stem cell pools outside this region have been iden-
tified in murine epidermis in recent years, suggesting that each
epidermal compartment is maintained in an autonomous fashion
(Page
et al.
, 2013; Kretzschmar and Watt, 2014). By transducing
epidermal cells with retroviruses encoding
β
-galactosidase, one of
these studies showed that the SG is maintained by a population of
long-lived progenitor cells from within or nearby the SG (Ghaziz-
adeh and Taichman, 2001). Later, Elaine Fuchs and co-workers pro-
posed the transcription factor B lymphocyte-induced maturation
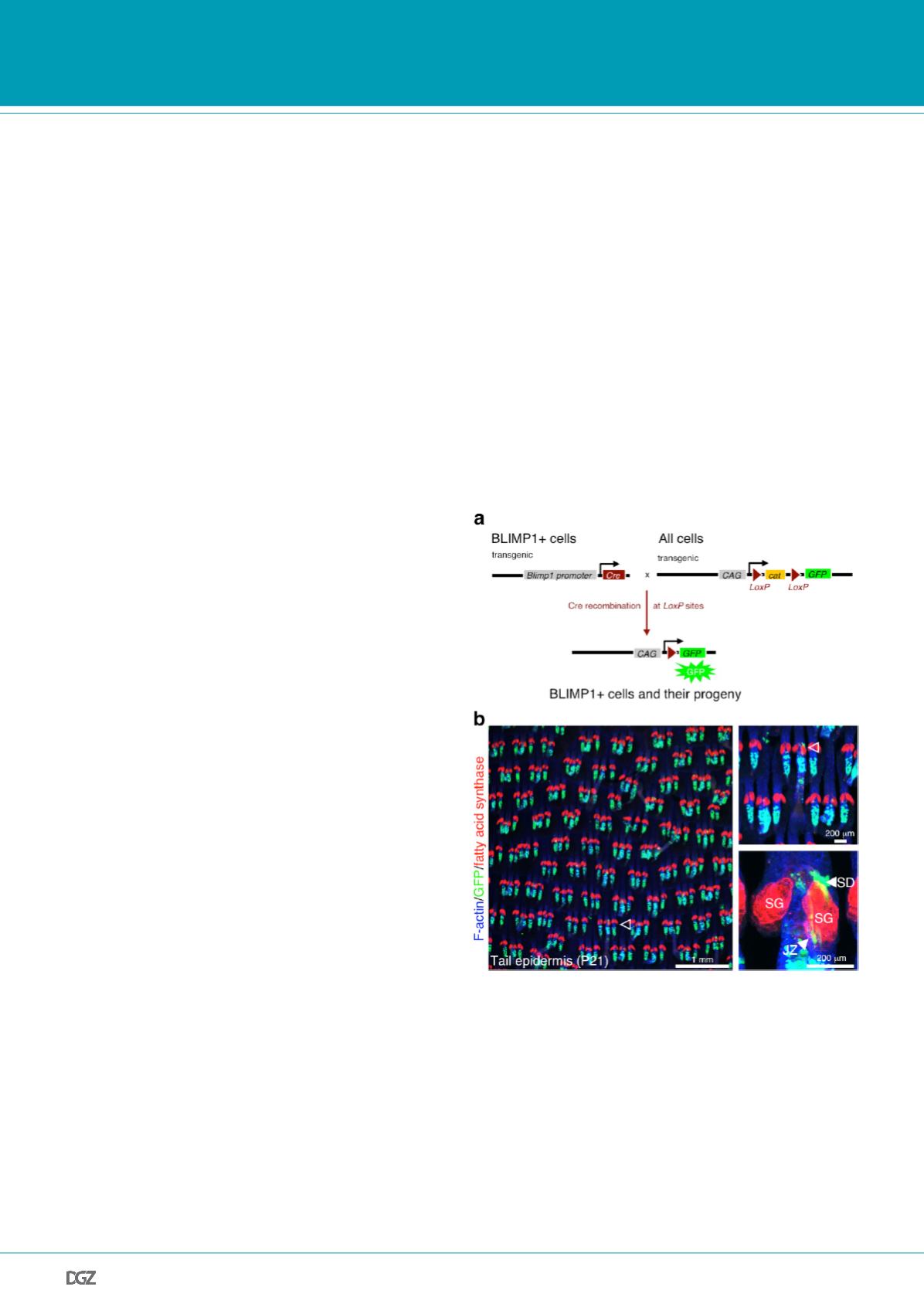
Figure 1: Genetic lineage
tracing of BLIMP1+ cells an
d their progeny
(a) Schematic of genetic lineage-tracing experiments.
Blimp1
Cre mice,
in which Cre recombinase is expressed under the control of the
Blimp1
promoter, are crossed with CAGcatGFP reporter mice. In
Blimp1
Cre ×
CAGcatGFP mice, Cre recombinase removes the floxed STOP (cat) cassette
in BLIMP1+ cells. This results in GFP expression in BLIMP1+ cells and,
subsequently, in all their progeny.
(b) Epidermal tail whole-mounts collected from
Blimp1
Cre × CAGcatGFP
mice at postnatal day (P) 21 stained with antibodies against GFP (green)
and fatty acid synthase (red) and counterstained with phalloidin (blue).
GFP-tracings were found in the hair follicle junctional zone (JZ) and
sebaceous duct (SD), but no GFP-labelled progeny of BLIMP1+ cells was
detectable in the sebaceous gland (SG).
Adapted from Kretzschmar
et al.
(2014).


