17
Cell News 2/2015
mitotic centrosomes. Hence, both, loss
of
CHK2-BRCA1
or overexpression of
AURKA
results in increased Aurora-A
activity at mitotic centrosomes, which
is a key trigger for increased micro-
tubule assembly rates. Consequently,
we showed that partial inhibition or
repression of
AURKA
restores proper
microtubule dynamics in CIN cells and
is sufficient to suppress CIN. However,
it is currently unclear how centroso-
me-based Aurora-A mediates an ac-
celeration of microtubule assembly
at microtubule plus ends, a question
that is currently addressed in our lab.
Furthermore, given the high frequency
of abnormal microtubule dynamics as
a trigger for CIN it is conceivable that
additional genes and pathways are
expected to contribute to this pheno-
type and we are currently focusing on
systematically identifying such regu-
lators to obtain a comprehensive view
on the causes of abnormal microtubu-
le assembly in CIN cells.
Mechanisms of chromosome
missegregation in response to
increased microtubule dynamics
Abnormal microtubule dynamics
might influence proper spindle for-
mation and, indeed, we found that
in cells with increased microtubu-
le assembly rates (induced either by
overexpression of
AURKA
or upon loss
of CHK2-BRCA1) mitotic spindle geo-
metry appeared abnormal (Fig. 3). In
particular, we found that those spind-
les were frequently mispositioned in
early mitosis, but not in metaphase
and this was suppressed when normal
microtubule dynamics was restored.
These transient spindle geometry and
positioning alterations are remotely
reminiscent to the transient multi-
polar spindle intermediates that are
observed in cells with supernumerary
centrosomes. Since the latter causes an increase in merotelic ki-
netochore attachments and the generation of lagging chromo-
somes, we evaluated whether these phenotypes are also indu-
ced upon the induction of increased microtubule growth rates.
Indeed, either overexpression of
AURKA
or loss of
CHK2-BRCA1
clearly caused the generation of hyper-stable erroneous micro-
tubule-kinetochore attachments and the generation of lagging
chromosomes during anaphase, both of which were suppressed
after restoration of normal microtubule growth rates in mitotic
cells. Together, these findings support the idea that increased
microtubule assembly rates cause abnormal spindle geometry
and orientation, possibly through deregulation of astral mi-
crotubules. This, in turn, facilitates the excessive formation of
merotelic kinetochore attachments leading subsequently to
chromosome missegregation (summarized in Fig. 4). However, so
far it is unknown why microtubule plus end dynamics is crucial
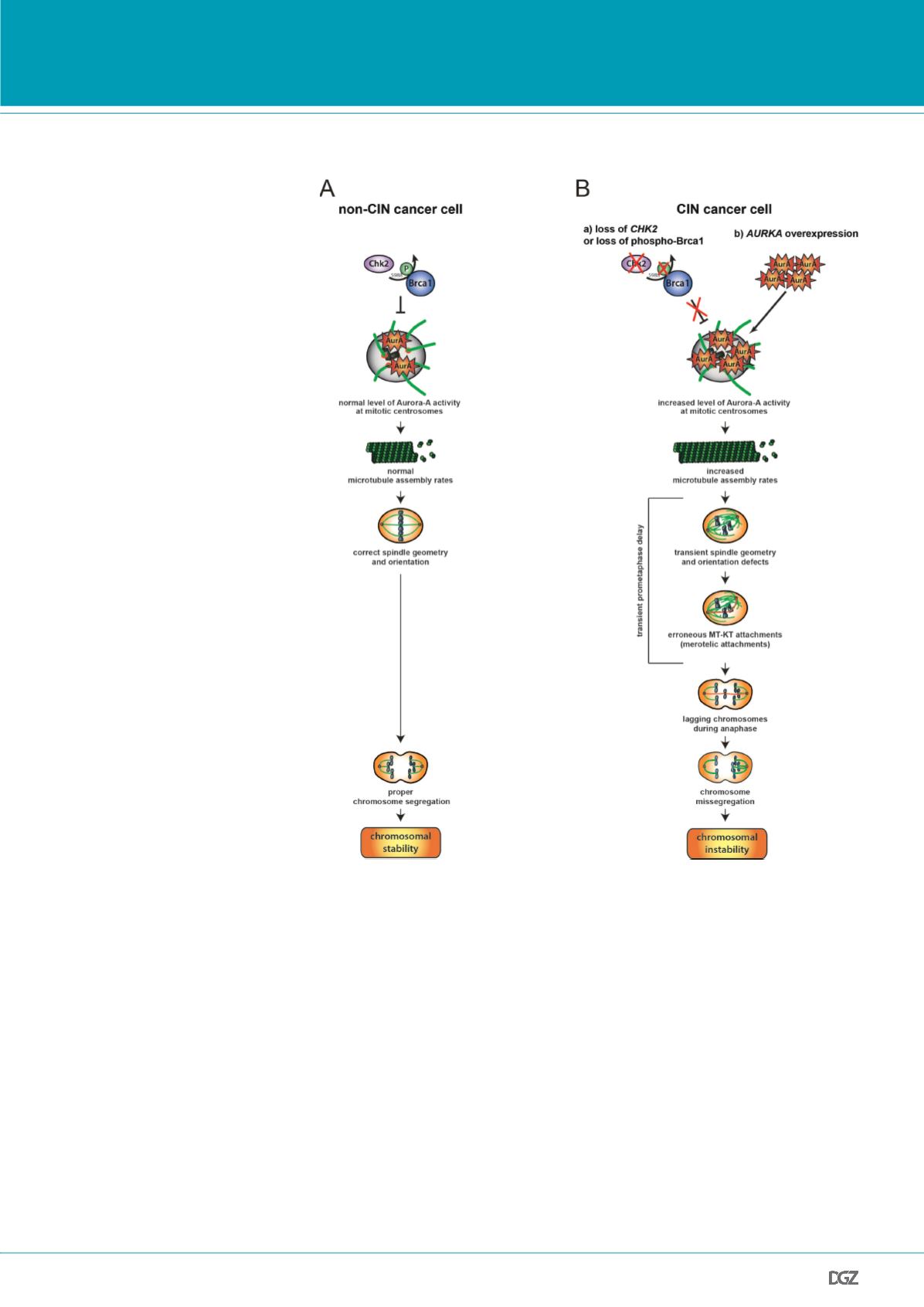
Figure 4:
Model summarizing the role of altered microtubule plus end assembly in chromosome misse-
gregation in cancer cells. (A) In normal or non-CIN cancer cells proper levels of centrosomal Aurora-A
is ensured by the Chk2-Brca1 pathway and is required for maintaining proper microtubule plus end
growth rates. This, in turn, is essential for proper chromosome segregation. (B) In cancer cells exhibi-
ting CIN either loss of the Chk2-Brca1 axis or overexpression of AURKA results in increased Aurora-A
kinase activity at mitotic centrosomes. This triggers an increase in microtubule plus end assembly, which
causes transient spindle geometry and positioning defects and facilitates the generation of erroneous
microtubule-kinetochore attachments. Unresolved merotelic kinetochore attachments result in lagging
chromosomes during anaphase and leads subsequently to chromosome missegregation constituting the
CIN phenotype. From: Ertych
et al.
, 2014.
PRIZE WINNERS


