10
Cell News 2/2015
PRIZE WINNERS
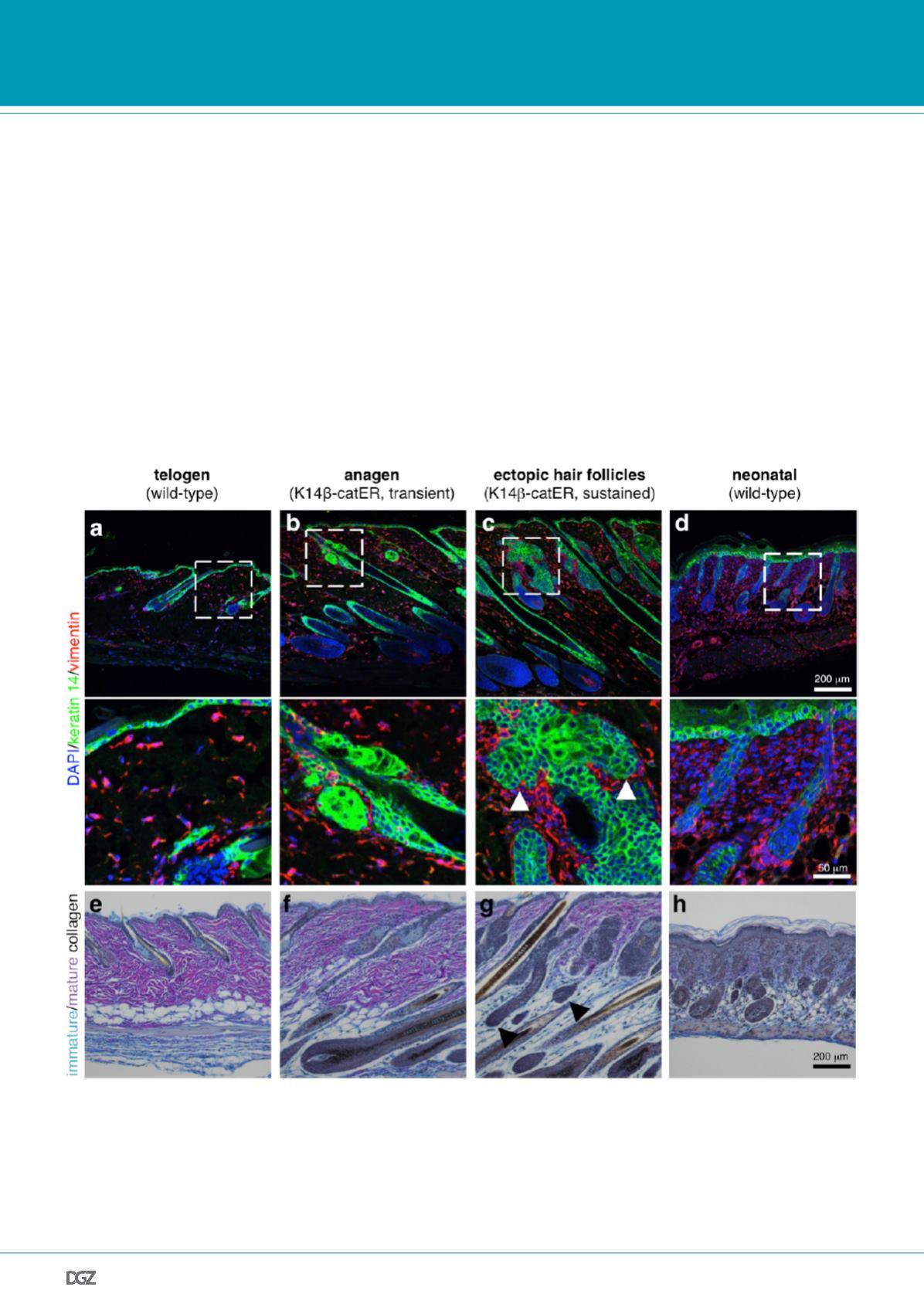
Figure 3: Sustained epidermal
β
-catenin activation reprograms adult dermis to a neonatal-like state.
Paraffin sections of back skin collected from adult wild-type mice (a, e); adult K14
β
-catER mice, which were treated with one dose of 4-hydroxytamoxifen
to allow transient
β
-catenin activation triggering hair follicle growth (anagen; b, f), adult K14
β
-catER mice, which were treated with six doses of 4-hydro-
xytamoxifen for two weeks to allow sustained
β
-catenin activation triggering ectopic hair follicle formation (c, g) and neonatal wild-type mice (d, h).
(a-d) Sections were labelled with antibodies against keratin 14 (green) and vimentin (red). Nuclei were counterstained with 4',6-diamidino-2-phenylindole
(DAPI). Inserts indicate higher magnifications shown below. Arrowheads indicate areas of ectopic hair follicle formation.
(e-h) Sections were stained following Herovici’s method to visualise thick, mature collagen fibres (pink) and immature collagen fibrils (bright blue). Nuclei
are stained grey/blue. Black arrowheads indicate ectopic hair follicles.
Adapted from Collins
et al.
(2011).
using a transgenic mouse line (K14
β
-catER) can also induce new
HF-like structures from IFE and SG (termed ectopic HFs), which are
associated with ectopic DPs (Lo Celso
et al.
, 2004). DPs normally
do not form
de novo
in adult skin. This result therefore suggested
that epidermal keratinocytes can remodel their dermal niche (Fig.
2). We hypothesised that the characteristics of dermal fibroblasts
change during postnatal development depending on whether hair
formation is induced or prevented by molecular alterations.
Due to a lack of fibroblast-specific markers these epidermal-der-
mal interactions have not been studied intensively. We have esta-
blished that platelet-derived growth factor receptor
α
(PDGFR
α
) is
expressed by all dermal fibroblasts in embryonic and postnatal skin
(Collins
et al.
, 2011). Using a mouse reporter line that expresses
green fluorescent protein (GFP) under the control of the
Pdgfra
promoter, we were therefore able to isolate dermal fibroblast as
well as epidermal keratinocytes and subsequently performed mic-
roarray analysis. This gene expression profiling revealed that der-
mal fibroblasts are reprogrammed into a neonatal-like state upon
ectopic HF formation. Sustained epidermal
β
-catenin activation
stimulated dermal fibroblasts to proliferate and remodel the extra-
cellular matrix (Fig. 3). In particular areas surrounding ectopic HFs
forming from the SG showed high fibroblast density and deposition
of immature collagen, which is typically only found in neonatal
skin. Our results demonstrated that the adult dermal niche of epi-
dermal stem cells is unexpectedly plastic and can be reprogram-
med into a neonatal-like state in response to epidermal cues, such
as
β
-catenin activation (Collins
et al.
, 2011).


