Cell News 3/2013
21
complexes
19
. In contrast, the membrane ATPase Pma1 has been
shown to occupy a network-like domain
18
(MCP: membrane
compartment occupied by Pma1). Dynamic, patch-like domains
were also described for Tor Complex 220 and endocytic actin
patches
21
.
In this regard, we have recently shown that all proteins in the
yeast PM are likely organized into distinct domains or patterns of
variable density (Figure 2), and that they exhibit unusually slow
diffusion rates
15
. Most of the tested proteins either segregated
from each other (Figure 3A) or only overlapped randomly15 (Fig-
ure 3B). The observed patterns were sensitive to cellular lipid
composition and the degree of colocalization between integral
membrane proteins was dependent on the similarity of their
membrane anchors
15
. We were even able to redirect proteins to
new locations by simply swapping their TM region
15
. We also
showed that a particular domain association can be essential
for the biological function of PM proteins
15
. In summary, our
findings indicate that proteins in the yeast PM self-organize
into numerous partially overlapping domains. Such a patchwork
membrane (Figure 4) likely arises from a combination of weak
interactions between the diverse lipid and protein constituents
of biological membranes.
Cortical acto-myosin
dynamics
A direct effect of the cortical ac-
tin cytoskeleton as PM-associated
fence that restricts lateral mobili-
ty of PM proteins has been shown
for mammalian cells
12,13
. Similarly,
cortex organization of plant cells
has been proposed to be partially
driven by cortical microtubule ar-
rays
22
. In our lab, using the Lifeact
marker that we previously devel-
oped
23,24
, we have started to inves-
tigate how the apical cell cortex
of epithelial cells is organized by a
pulsatile acto-myosin network. In
yeast cells, cortical actin cables are
quite sparse and show very fast dynamics with individual cables
being translated along the PM by the type V myosin Myo2 with
up to 3 μm/s
25,26
. Considering the slow diffusion of TM proteins,
actin in yeast is therefore not expected to directly influence la-
teral segregation of the PM. Indeed, removal of all actin had only
minor effects on yeast PM domain organization
15
.
On the other hand, actin is a critical compo-nent for both en-
docytosis and exocytosis, which in turn control the continuous
turnover of PM proteins and lipids. The temporal and spatial or-
ganization of actin-mediated membrane delivery and internali-
zation is therefore of central importance for our understanding
of membrane patterning. In particular, positioning of slowly dif-
fusing TM proteins is expected to critically depend on the distri-
bution of entry and exit sites.
The role of the cell wall
A striking feature of the yeast PM is the slow diffusion of its
constituents. Many TM proteins move with diffusion rates that
are several orders of magnitude lower than those of comparable
proteins in mammalian system
15,38
. One possible explanation for
this striking behavior is that many proteins in the yeast PM have
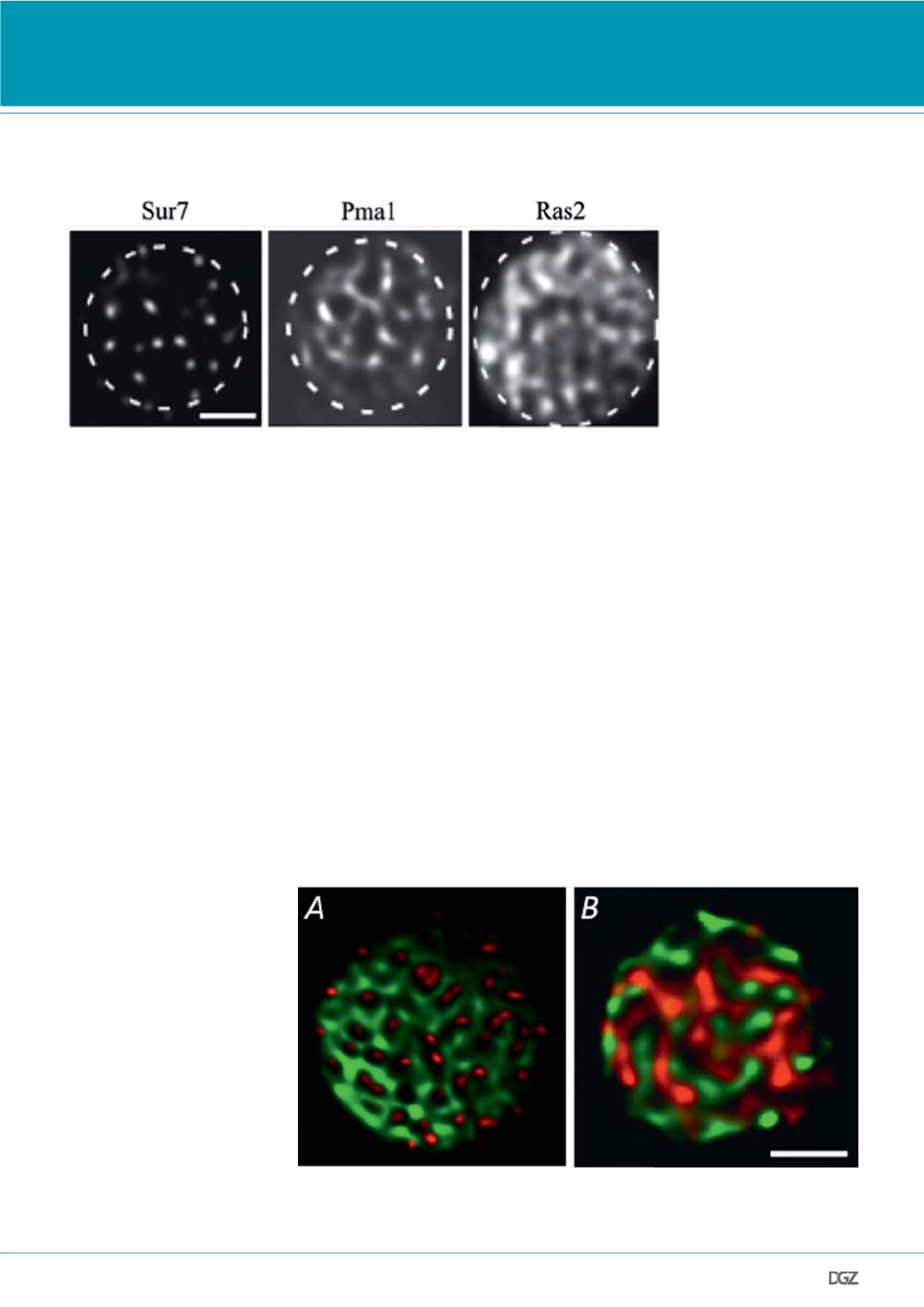
Figure 2.
Domain formation of GFP-labeled
yeast PM proteins. The selected
TIRFM images demonstrate the
observed density range from patch-
to network-like domains. Scale bar:
2 μm. Adapted from (15).
Figure 3.
Segregation of yeast PM proteins. Shown are two color TIRFM examples of (A) complete segregation (Pma1-
GFP and Sur7-RFP) and (B) random overlap (Fet3-GFP and Pma1-RFP). Scale bar: 2 μm. (B) adapted from (15).
RESEARCH NEWS


