Cell News 2/2016
14
for vesicle movement. This was unexpected because long-range
transport of vesicles was generally thought to be microtubule-
dependent (Schuh, 2011).
Moreover, we found that the vesicles not only move along the actin
network, but that oocytes use the vesicles as adaptable, motorized
network nodes to regulate the dynamics and density of the net-
work. In particular, Rab11a-positive vesicles drive the network dy-
namics in a myosin-Vb-dependent manner, and help to modulate
the network density by sequestering and clustering the network's
actin nucleators. Our work also revealed a simple way by which
networks of different densities can be generated, namely by ad-
justing the number and volume of vesicles in the cell. This vesic-
le-based mechanism of actin network modulation is essential for
asymmetric positioning of the meiotic spindle in mouse oocytes
(Holubcova et al., 2013) (Fig. 2).
Meiotic spindle organization
Our work also focussed on how the acentrosomal meiotic spindle
is organized in mammalian oocytes. This topic is of particular rele-
vance because our work in human oocytes suggests that multipolar
spindle intermediates and spindle instability, which are a frequent
phenomenon during spindle assembly in human oocytes, correlate
with lagging chromosomes, which are a source of chromosome se-
gregation errors (Holubcova et al., 2015).
Spindle assembly in mouse oocytes is driven by self-organization
of numerous acentriolar microtubule organizing centers (MTOCs)
(Schuh and Ellenberg, 2007). Recent work from our lab has re-
vealed that mouse oocytes need to fragment the MTOCs into a high
number of small MTOCs to be able to then regroup and merge them
into two equal spindle poles. We found that MTOCs are fragmented
in a 3-step process. First, Plk1 triggers a loosening of MTOC struc-
ture that involves the release of the centrosomal linker protein C-
Nap1. Second, BicD2-anchored Dynein stretches the MTOCs into
fragmented ribbons on the surface of the nuclear envelope. Third,
KIF-11 further fragments the MTOCs following nuclear envelope
breakdown so that they can be evenly distributed towards the two
spindle poles. Blocking MTOC fragmentation leads to defects in
spindle assembly, which delay chromosome individualization and
congression (Clift and Schuh, 2015).
In collaboration with Takashi Hiiragi’s lab at EMBL Heidelberg,
we have also studied the mechanism of spindle assembly in early
mouse embryos. This work revealed a surprising gradual transition
from meiosis to mitosis over the first eight divisions of the mouse
embryo: spindle assembly in the mouse zygote is achieved by a
similar mechanism as in mouse oocytes, and only gradually, the
spindle adapts the properties of a centrosomal spindle found in
typical mitotic cells (Courtois et al., 2012).
Live imaging screen reveals causes of meiotic defects in
mammalian oocytes
To understand why human oocytes are so frequently abnormal we
need to identify the genes that safeguard accurate progression
through meiosis. To achieve this aim, we developed an experimen-
tal strategy that allowed us to carry out the first RNAi screen in
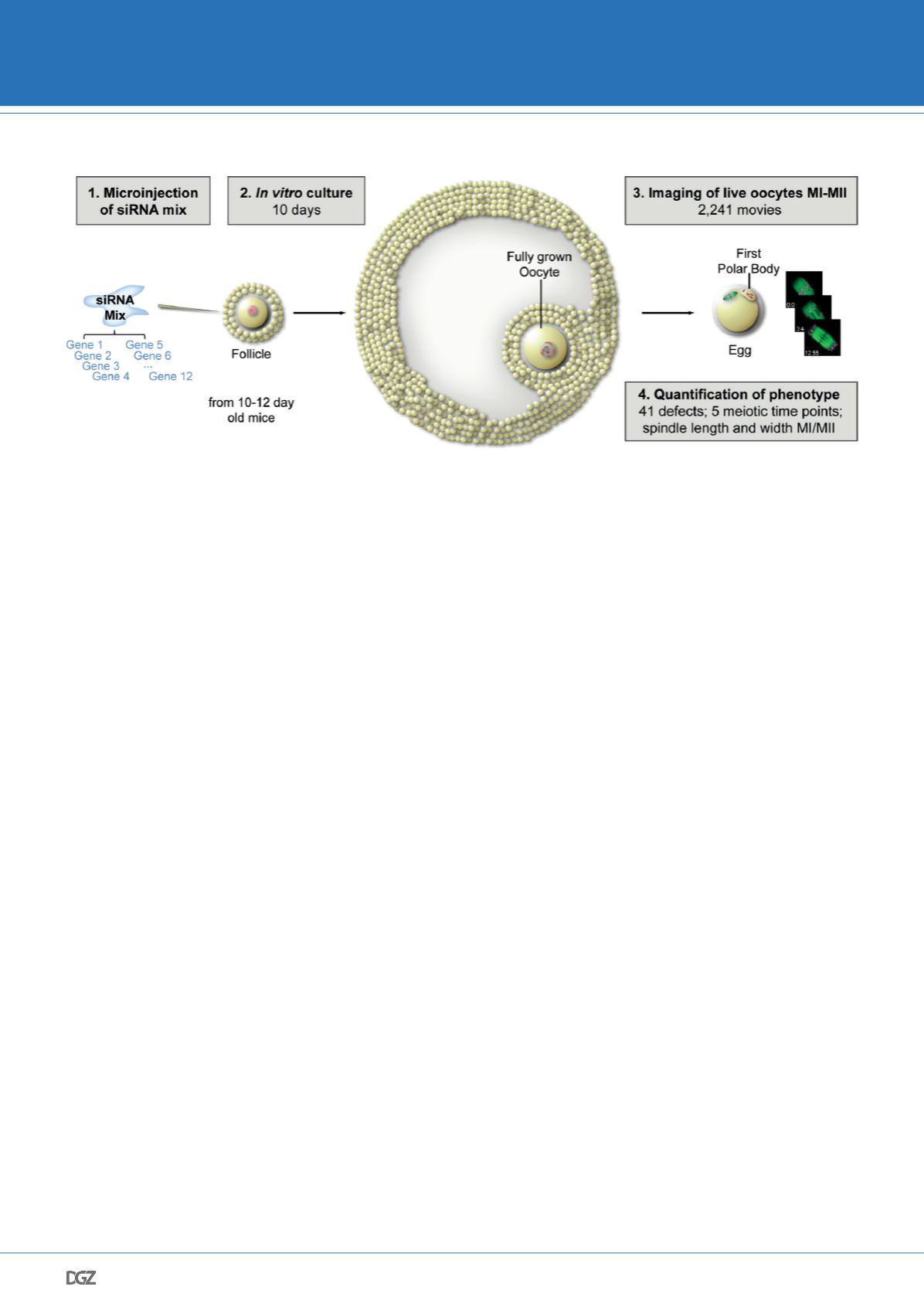
mammalian oocytes. (Fig. 3) We analyzed the function of 774 ge-
nes by high-resolution imaging of chromosomes and microtubules
during meiosis in live mouse oocytes and scored each oocyte quan-
titatively for 50 phenotypes, generating a comprehensive resource
of meiotic gene function. This resource allowed us to identify and
characterize many new genes essential for meiosis in mammalian
oocytes. The screen generated an unprecedented annotated data-
set of meiotic progression in 2,241 mammalian oocytes. This data-
set also allowed us to analyze systematically how meiotic defects
arise (Pfender et al., 2015).
Figure 3. Scheme illustrating principle of RNAi screen in mouse oocytes.
siRNAs targeting several genes simultaneously were microinjected into follicles, which were then cultured in vitro for 10 days. Chromosomes and microtu-
bules were imaged and phenotypes were assessed in more than 2,200 live oocytes.
© Pfender et al., Nature 2015
DGZ AWARD WINNERS 2016


