Cell News 2/2016
19
DGZ AWARD WINNERS 2016
tical interventions targeting these hormonal pathways. Therefore
there is a need to better understand the molecular basis control-
ling AgRP-neurons activity as discovery of new regulators involved
in the central control of energy balance and glucose homeostasis
will ultimately appear as potential novel therapeutic target for the
treatment of obesity and T2DM.
Among all potential novel regulators of the central control of appe-
tite, the family of G-protein-coupled receptors (GPCRs) carry great
therapeutic potential. Among them, the purinergic receptor family
represents one of the most abundant receptors in living organisms
and is highly conserved throughout evolution. A striking example
of the importance of purinergic signaling was the discovery of ATP
as a neurotransmitter regulating core physiological func-
tions, including feeding behaviour. Surprisingly, aside from
ATP, the involvement of nucleotides as extracellular signal
molecules in the central control of food intake is unknown.
Interestingly, members of the P2Y family, such as P2Y1 and
P2Y14, have previously been associated with homeostatic
processes such as food intake and insulin sensitivity (Burn-
stock et al., 2011). In our work, we find that the purinergic
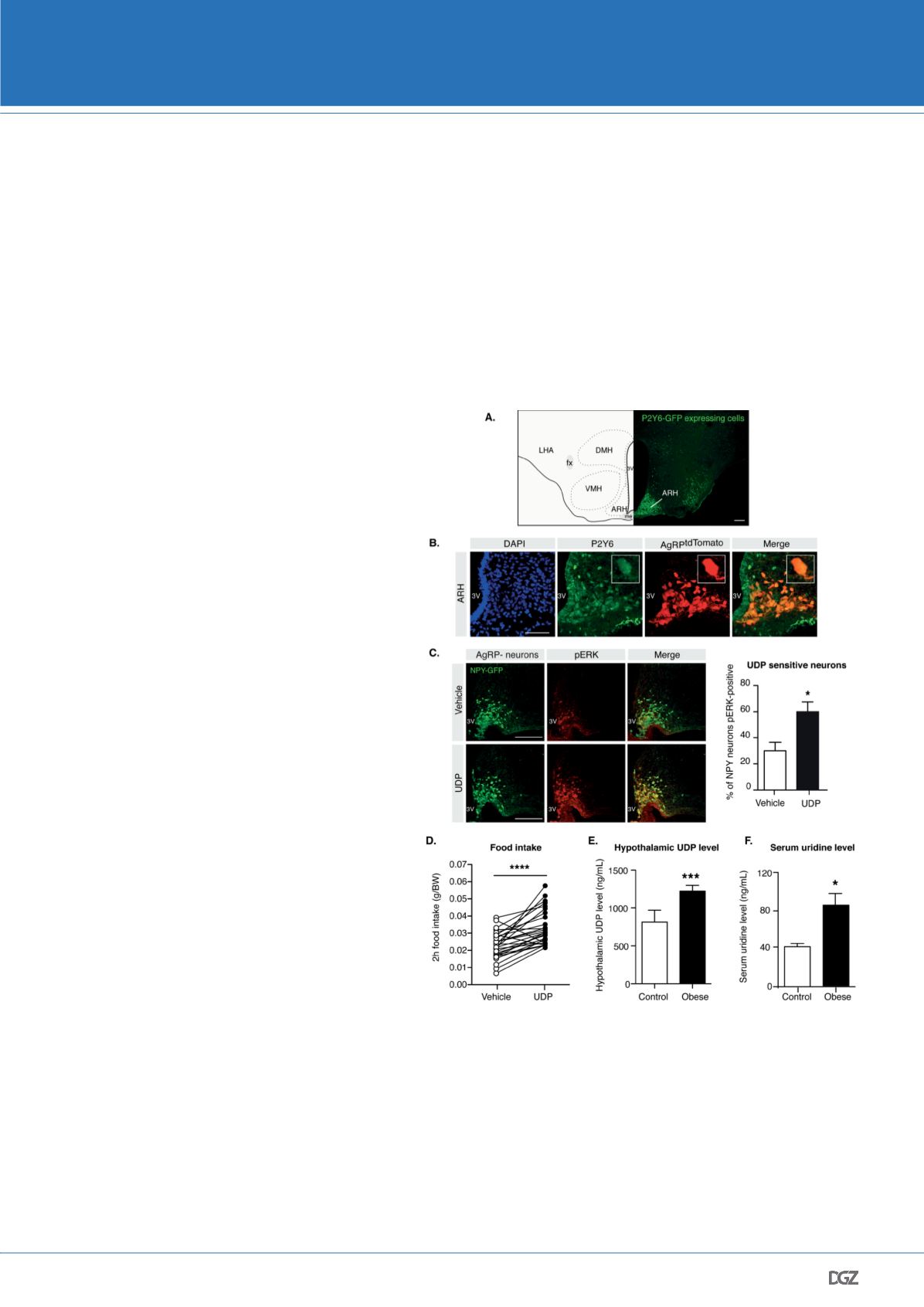
receptor 6 (P2Y6) is highly enriched in the ARH (Figure 2A)
and most importantly, that AgRP-neurons express P2Y6
(Figure 2B) (Steculorum et al., 2015). Having identified
that P2Y6 is expressed on AgRP-neurons, we next inves-
tigated whether application of the P2Y6 agonist uridine
diphosphate (UDP) directly into the brain could modula-
te activation of AgRP-neurons. The central application of
UDP was achieved by intracerebroventricular (icv) appli-
cation, in which the compound is directly administered
into the cerebrospinal fluid via cannulation of the lateral
ventricle. Mice expressing the green fluorescent protein
GFP in AgRP-neurons were implanted with icv cannulas.
Following icv administration of either vehicle (i.e. saline)
or UDP, brains were processed for immunohistochemistry
against the tyrosine phosphorylation of the MAP kinases
ERK-1 and ERK-2 (pERK), as P2Y6 is well known to activate
the ERK-signaling pathway. Quantification of pERK immu-
noreactivity revealed that UDP significantly increased the
number of pERK-positive AgRP-neurons (Figure 2C), re-
vealing that P2Y6 is expressed on those neurons and that
it is functional, as UDP directly activates its intracellular
signalling. We next aimed to further support the notion,
that the P2Y6 agonist UDP can directly modulate the acti-
vity of AgRP-expressing neurons. Thus, we performed per-
forated patch clamp recordings from mice expressing the
tomato reporter in AgRP-neurons in order to directly mea-
sure the impact of UDP on the electrophysiological pro-
perties of AgRP-neurons. Here, we found that application
of UDP directly to AgRP-neurons resulted in an increased
action potential frequency of these cells, which translate a
direct activation of those neurons by UDP.
In light of the pivotal role of AgRP-neuron activation in
the orexigenic effect of UDP, we next wondered whether
the hereto newly identified activator of these neurons, was
indeed able of increasing feeding. Therefore, control mice
were icv injected with UDP before the onset of the dark
period, i.e. when spontaneous feeding occurs in mice. We found
that central application of UDP indeed acutely enhanced sponta-
neous food intake (Figure 2D).
Having identified a novel regulatory pathway controlling AgRP-
neuron activity and feeding, we next investigated whether this
pathway could be deregulated in obese and diabetes conditions.
One of the key critical finding arising from these investigations was
that hypothalamic UDP concentrations are significantly increased
in diet-induced obese mice (i.e. mice fed a high fat diet) (Figure
2E). Neuronal UDP synthesis critically relies on the availability of
uridine, which originate from the periphery and reaches the brain
to serve as a precursor metabolites to synthetize UDP. We there-
Figure 2: P2Y6 and its ligand UDP are novel regulators of AgRP-neurons activity
and food intake.
Representative microphotograph of A. GFP immunostaining (P2Y6-GFP, green) in
the medio-basal hypothalamus (ARH, arcuate nucleus of the hypothalamus; LHA,
lateral hypothalamic area; VMH, ventromedial nucleus of the hypothalamus; DMH,
dorsomedial nucleus of the hypothalamus; fx, fornix; 3V, third ventricle; me, median
eminence) and B. P2Y6 (green) and AgRPtdTomato-neurons (AgRPtdTomato, red) in
the ARH. C. Confocal images and quantitative comparison of NPY-GFP expressing
neurons (i.e AgRP-neurons) pERK immunoreactive after intracerebroventricular (icv)
administration of vehicle (saline) or UDP. Scale bar: A and C: 100 μm; B: 50 μm. D. 2
hours food intake following icv administration of vehicle or UDP depicted as paired-
wise analysis of individual mice that received both vehicle and UDP. E. Hypothalamic
contents and F. circulating uridine levels of control and obese mice. Adapted from
Steculorum et al., 2015.


