Cell News 2/2016
15
DGZ AWARD WINNERS 2016
Studying meiosis and causes of aneuploidy in human
oocytes
Most of our knowledge about aneuploidy in mammalian oocytes
stems from studies in mouse oocytes. However, the relevance of
this work for human aneuploidy is often unclear because we still
know very little about meiosis in human oocytes. Studies of mei-
osis in live human oocytes for instance that could reveal the cau-
ses of aneuploidy were completely missing. Our lab has pioneered
methods that facilitated the first studies of meiosis and causes of
aneuploidy directly in live human oocytes.
We have been able to record videos of spindle assembly and chro-
mosome segregation in more than 100 human oocytes. These vi-
deos allowed us for the first time to establish the different stages
through which meiosis progresses in human oocytes (Fig. 4). They
also provided exciting new insights into the causes of chromoso-
me segregation errors in human oocytes. We found that human
oocytes often assemble a bipolar spindle by progressing through a
prolonged multipolar spindle stage. Oocytes progressing through
this stage are particularly likely to have lagging chromosomes in
anaphase, a phenomenon that may be facilitated by a large num-
ber of abnormal kinetochore microtubule attachments in human
oocytes. Thus, our data suggest that spindle instability and transi-
ent multipolarity contribute to the high frequency of chromosome
segregation errors in human oocytes, even in young women (Ho-
lubcova et al., 2015).
Our work also shed light on why aneuploidy in human oocytes in-
creases with maternal age. We found that many sister kinetochores
in human oocytes are separated and do not behave as a single
functional unit during the first meiotic division. Having separated
sister kinetochores allows bivalents, the unit of two homologous
chromosomes linked to each other by recombination, to rotate by
90 degrees on the spindle and increased the risk of merotelic kine-
tochore-microtubule attachments (Fig. 5). Advanced maternal age
led to an increase in sister kinetochore separation, rotated biva-
lents and merotelic attachments. Chromosome arm cohesion was
weakened, and the fraction of bivalents that precociously dissoci-
ated into univalents was increased. Together, these data suggest
that multiple age-related changes in chromosome architecture
contribute to the increase of oocyte aneuploidy with advanced
maternal age (Zielinska et al., 2015).
Outlook
Despite decades of work, we still know relatively little about mei-
osis in mammalian oocytes. Especially human oocytes have hardly
been studied, which is surprising given that all our lives started
with the fertilization of an egg. Fertility problems become more
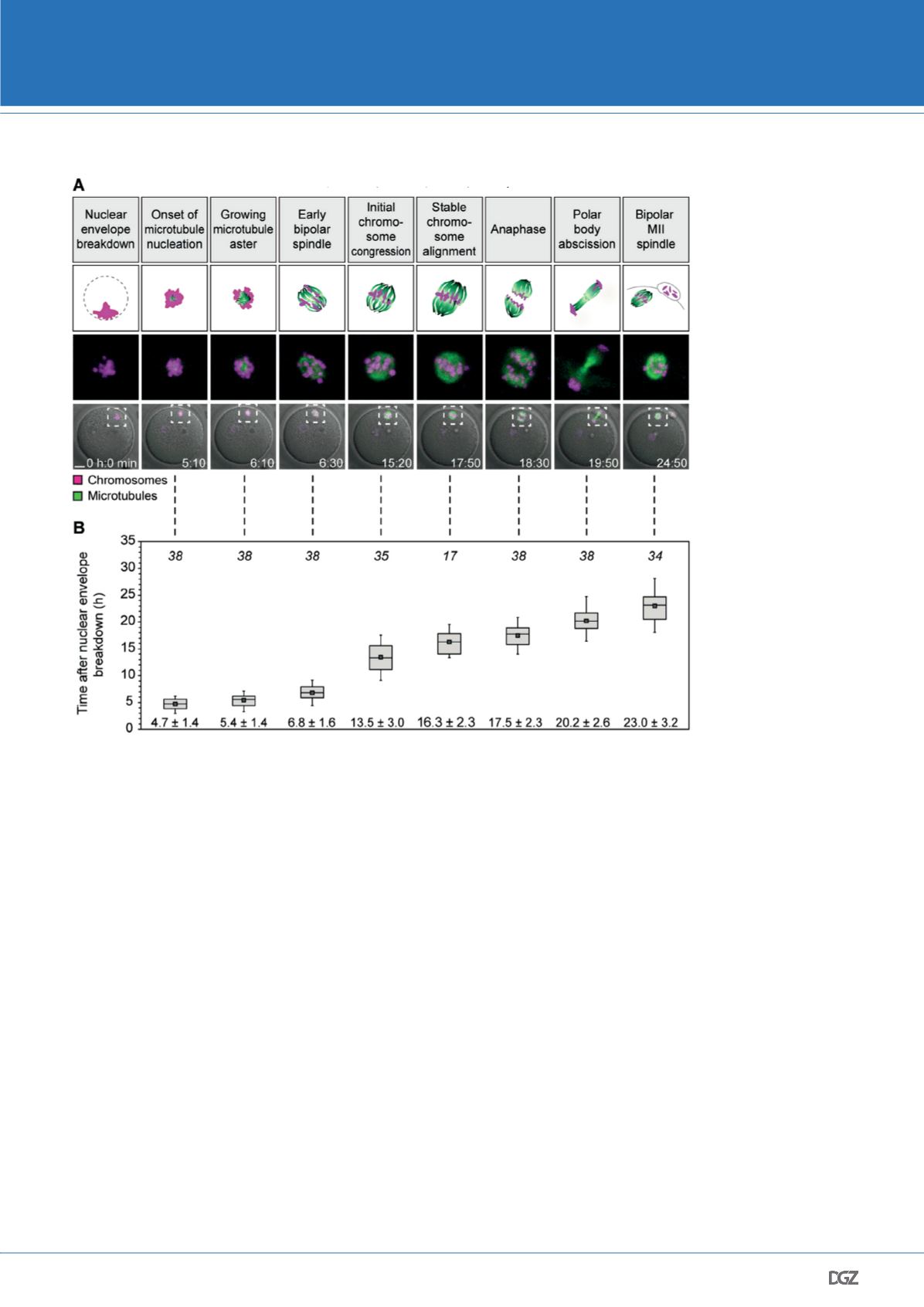
Figure 4. Stages and timing of
meiosis in human oocytes.
(A) Stages of meiosis in human
oocytes determined from live
human oocytes expressing
EGFP-MAP4 (microtubules) and
H2B-mRFP1 (chromosomes).
A schematic representation of
each stage (scheme; microtu-
bules in green; chromosomes
in magenta) and stage-specific
time-lapse images (z-pro-
jections, 4 sections, every 5
μm) merged with differential
interference contrast [DIC] are
shown (bottom row). Outlined
regions are magnified above
(middle row). Scale bar, 20 μm.
Time displayed in hours: minu-
tes. (B) Quantification of timing
of meiotic progression from live
oocytes expressing EGFP-MAP4
(microtubules) and H2B-mRFP1
(chromosomes) as shown in
(A). The box plot shows median
(line), mean (small square), and
25th and 75th (boxes), 5th and
95th percentile (whiskers) of
time after NEBD. The number of
oocytes is specified in italics.
© Holubcova et al., Science
2015


