Cell News 2/2016
20
fore studied if the increased hypothalamic UDP-levels observed in
obesity is associated with an increase of circulating uridine. We
found that circulating uridine is increased in obese and diabetic
mice and that hypothalamic UDP-levels are therefore positively
correlated with circulating uridine (Figure 2F). Taken together, our
work revealed that in obesity circulating uridine concentrations are
increased, providing enhanced substrate availability for hypothala-
mic UDP-synthesis, ultimately promoting feeding via UDP-induced
P2Y6 signaling in the CNS.
In summary, in this study, we identified the UDP/P2Y6-axis as a
novel regulator of AgRP-neuron activity and feeding
behaviour. This offers the unique opportunity to pur-
sue agonists and antagonists for this GPCR as novel
targets for the treatment of diseases associated with
negative or positive energy balance.
Deciphering the glucoregulatory effects of
AgRP-neurons
In addition to their crucial role in controlling feeding,
AgRP-neurons have been implicated in the long-term
regulation of peripheral glucose homeostasis (Konner
and Bruning, 2012; Vogt and Bruning, 2013). From
an evolutionary aspect, it is reasonable to hypothe-
size that the same neurons, which control uptake of
nutrients from the environment according to energy
status of the organism, also control nutrient fluxes
within the body. However, experiments suggesting a
role for AgRP-neurons in glucose homeostasis were
all based on models with chronic alterations of sig-
nalling pathways in AgRP-neurons, which are often
associated with obesity and therefore generate con-
founding effects. Thus, it remained unknown whether
acute activation of AgRP-neurons could control acu-
te glucose- and insulin sensitivity-regulatory effects.
In order to bypass secondary effects of chronic AgRP-
neurons manipulations, we took advantage of recent
technology that allows to acutely and remotely con-
trol neuronal activity of a molecularly defined neuro-
nal population. Our work was notably based on two
approaches: the chemogenetic and the optogenetic,
in which neuronal activity is respectively modulated
via the administration of a compound or exposure
to light (Sternson et al., 2016). Chemogenetic tech-
nology, also called Designer Receptors Exclusively
Activated by Designer Drugs (DREADD) relies, in our
study, on the use of a mutated form of the human
stimulatory muscarinic receptor (hM3DGq), which
has been mutated in order to block its activation by
endogenous ligands (i.e acetylcholine), but instead
recognizes an inert compound: clozapine-N-oxide
(CNO) (Figure 3A). In our laboratory, we enginee-
red hM3DGq-floxed mice for selective expression of
the hM3DGq-receptors in AgRP-neurons upon Cre-
recombination (Steculorum et al., 2016). The opto-
genetic technique is based on the properties of the
channelrhodopsin 2, a light-gated ion channel known
to activate the cells upon stimulation with a blue light (473 nm)
(Figure 3B). We also used the Cre-LoxP recombination system to
specifically express ChR2 in AgRP-neurons so that upon light sti-
mulation via an optic fiber placed stereotaxically in the appropriate
brain region, either directly at the levels of the soma or in distinct
projection sites, AgRP-neurons will selectively and specifically be
activated. We therefore used those two models to investigate the
influence of acute activation of AgRP-neurons in systemic insulin
sensitivity. Sensitivity to insulin was evaluated by challenging the
mice to an insulin tolerance test (ITT) in which mice received an
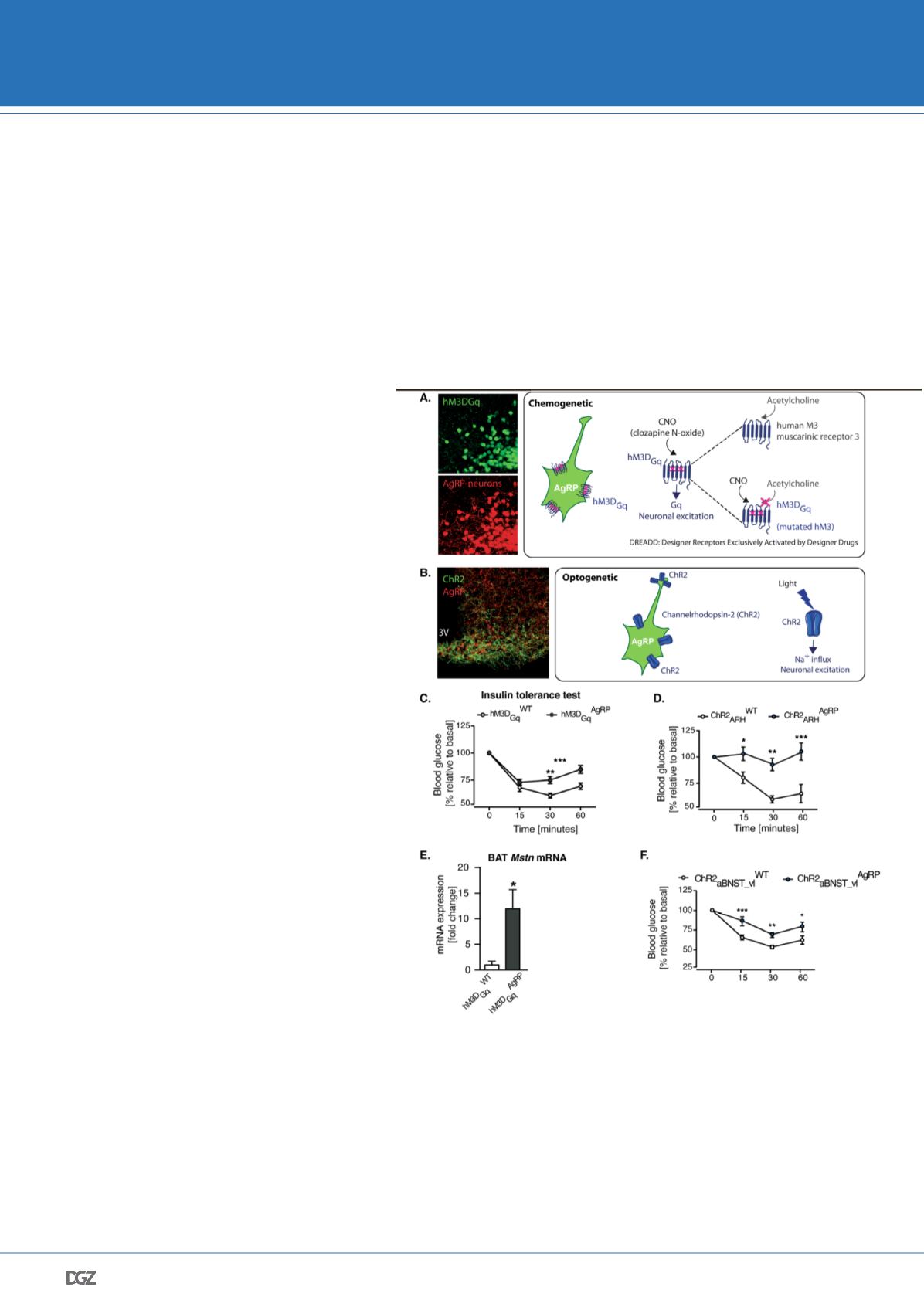
Figure 3. Acute activation of AgRP-neurons induced insulin resistance via increa-
sed myostatin expression in the brown adipose tissue.
Schematic illustrating A. the
chemogenetic and B. the optogeneic approaches used to acutely and remotely control the
activity of AgRP-neurons. Representative microphotographs show in A. the expression of
hM3DGq (green) in AgRP neurons (red) and in B. the expression of ChR2 (green) in AgRP
fibers (red). Insulin tolerance test (ITT) in (C) hM3DGqAgRP and control mice (i.e chemo-
genetic acute activation of AgRP-neurons) and (D) during somatic stimulation of ARH
AgRP neurons (ChR2ARHAgRP) and control litter mates (ChR2ARHWT) (i.e. optogenetic
activation of AgRP-neurons). E. qRT-PCR analysis of Myostatin (Mstn) mRNA expression
in the brown adipose tissue of hM3DGqAgRP and hM3DGqWT in mice 1 hr after CNO in-
jection. F. ITT in ChR2AgRP mice and ChR2WT mice in which AgRP projections specifically
innervating the ventro-lateral anterior bed nucleus of the stria terminalis (aBNST_vl) have
been laser-light stimulated. Adapted from Steculorum et al., 2016.
DGZ AWARD WINNERS 2016


