Cell News 2/2016
18
Nikon Young Scientist Award of the DGZ:
Sophie Steculorum
Over the past decades, the concomitant apparition of Western diet
(i.e. high-fat, high-sucrose diet) and sedentary life style has led
to a dramatic rise in the prevalence of obesity and its associa-
ted metabolic diseases such as type 2 Diabetes Mellitus (T2DM)
(Geiss et al., 2014; WHO, 2006). Given the ever-increasing burden
of this present epidemic, there is an emergent need to better un-
derstand the mechanisms and factors involved in the development
of this pathological condition. Despite a clear improvement of our
understanding of how an organism maintains body weight and
blood glucose levels, fundamental knowledge regarding the exact
homeostatic mechanisms involved in the regulation of appetite and
glycemic control remain poorly understood. Nonetheless, extensive
research over the last several decades provided important advances
highlighting the critical importance of the central nervous system
(CNS) in control of energy balance and glycemic control.
The CNS as a key player in feeding and systemic glucose
regulation
More than one century ago, the French physiologist Claude Bernard
postulated that the CNS plays a key role in the control of peripheral
glucose metabolism, notably based on the observation that lesions
at the levels of the floor of the fourth ventricle in rabbits lead to
glycosuria (Bernard, 1855). He further proposed the existence of a
brain-periphery loop controlling systemic glucose levels. In parallel,
the observation that tumor development in the pituitary and the
hypothalamus lead to obesity and hyperphagia also drew atten-
tion to the importance of the brain in appetite and body weight
regulation (Babinski, 1900). Following these pioneer discoveries,
lesions-based experiments corroborated the critical role of the CNS
in control of body weight, appetite and glucose levels and allowed
to further define specific regions of the brain involved in these pro-
cesses. Especially, in the 1940’s, Hetherington and Ranson revealed
that the lesions of the medio-basal hypothalamus (Hetherington
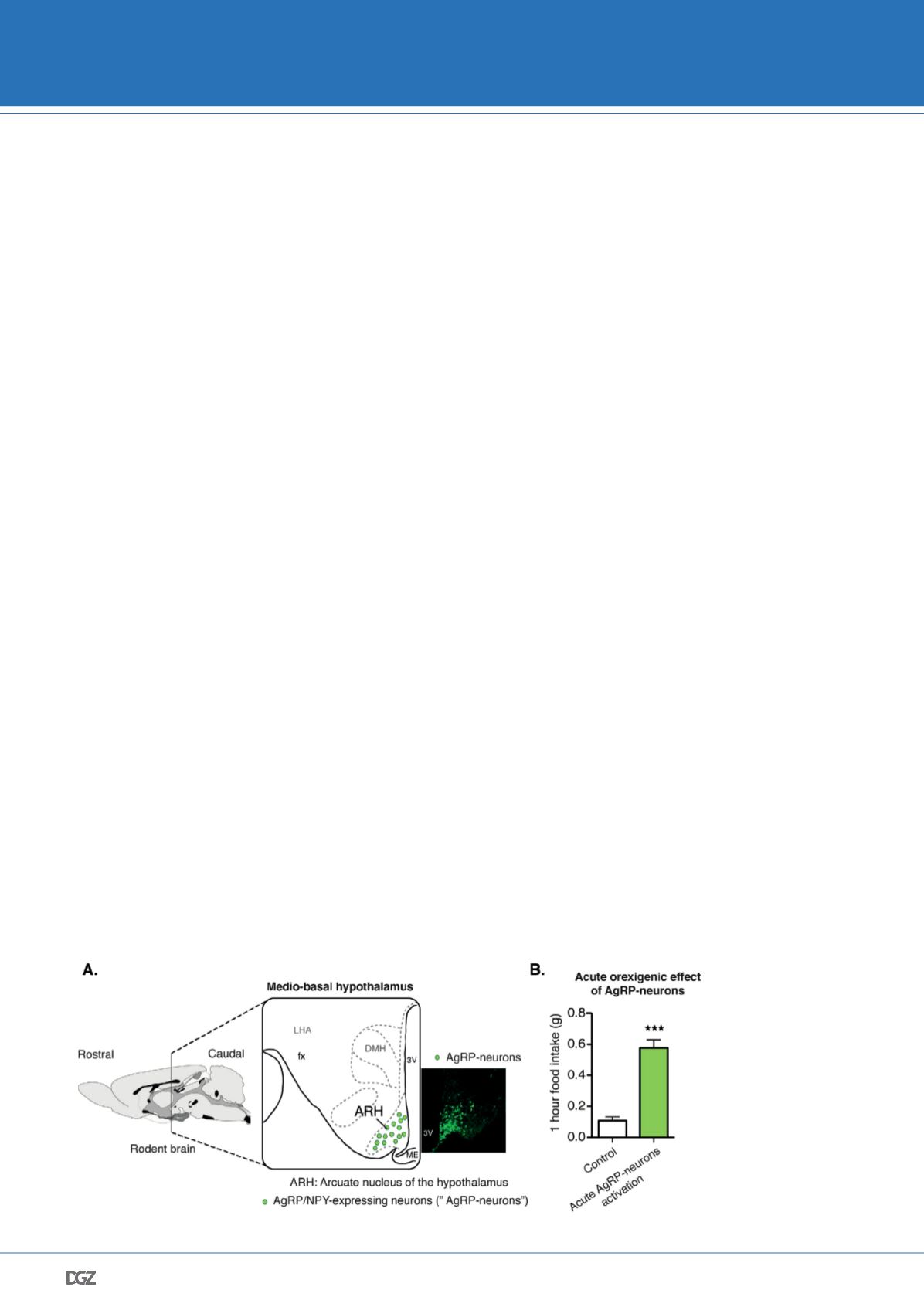
and Ranson, 1940) (Figure 1A), which notably contains the arcu-
ate nucleus of the hypothalamus (ARH), lead to obesity. The ARH
contains neurons located in the ventral part of the ARH (Figure
1A) that co-express two orexigenic peptides: neuropeptide Y (NPY)
and agouti-related peptide (AgRP) (for the following part, those
neurons will be called: AgRP-neurons) (Vogt and Bruning, 2013).
AgRP-neurons have primarily been studied for their well-described
orexigenic effect and their role in feeding and body weight regula-
tion. Notably, acute activation of AgRP-neurons leads to voracious
feeding, illustrated by a 5 fold increase in food intake within an
hour (Figure 1B) (Steculorum et al., 2016). Moreover, ablation of
AgRP-neurons in adult mice leads to death secondary to cessation
of feeding clearly highlighting their critical importance in feeding
regulation (Luquet et al., 2005).
Discovering new modulator of appetite by identifying
novel regulators of AgRP-neurons activity.
Shortly after the discovery of the critical importance of AgRP-
neurons in feeding and body weight regulation, it was shown that
obesity and T2DM are associated with the onset of a neuronal re-
sistance to the two main regulators of those neurons (Friedman,
2004; Konner and Bruning, 2012). Indeed, while a lot of studies
investigated the role of leptin and insulin (respectively secreted by
the white adipose tissue and the pancreas) in the control of AgRP-
neurons activity under lean conditions,
the discovery that AgRP-neurons be-
come insulin- and leptin- resistant
upon development of obesity and T2DM
conditions clearly limits pharmaceu-
Figure 1: Neuroanatomical
location of AgRP-neurons
and their orexigenic effect.
A. Schematic representing
the location of neurons
co-expressing Agouti-
related peptides (AgRP)
and neuropeptide Y (NPY)
neurons (called AgRP-
neurons). AgRP-neurons
are located in the arcuate
nucleus of the hypothala-
mus (ARH), more specifically
in the medio-basal region
of the hypothalamus. The
microphotograph shows an
example of AgRP-neurons’
location in mice expressing
green-fluorescent protein
in AgRP-neurons (here, in
NPY-GFP mouse). B. Acute
activation of AgRP-neurons
promotes feeding, adapted
from Steculorum et al.,
2016.
DGZ AWARD WINNERS 2016


