Cell News 2/2016
16
and more prominent in our society. Many women in the Western
world decide to have a career and are forced to postpone childbe-
aring until natural conception becomes difficult or impossible. To
improve fertility treatments it is essential that we better under-
stand the mechanisms that govern accurate progression through
meiosis and that we analyse the causes of chromosome segrega-
tion errors in mammalian oocytes. Thus, research into mammalian
oocytes, with its many open questions and medical implications,
has enormous potential to grow and will remain an attractive field
for a long time to come.
Acknowledgements
I would like to thank all previous and current members of my labo-
ratory for their fantastic work and commitment to their projects. I
would also like to thank the great mentors that have supported me
throughout the different stages of my scientific career and shaped
my scientific thinking, including Stefan Heidmann, Christian Leh-
ner, Jan Ellenberg, Matthew Freeman and Sean Munro. The re-
search leading to these results has received financial support from
the Medical Research Council, the Max Planck Society, the Euro-
pean Research Council under grant agreement no. 337415, the Eu-
ropean Community’s Seventh Framework Programme (FP7/2007-
2013) under grant agreement no. 241548 as well as from the Lister
Institute of Preventive Medicine, the Rosetrees Trust, EMBO and
Boehringer Ingelheim Fonds.
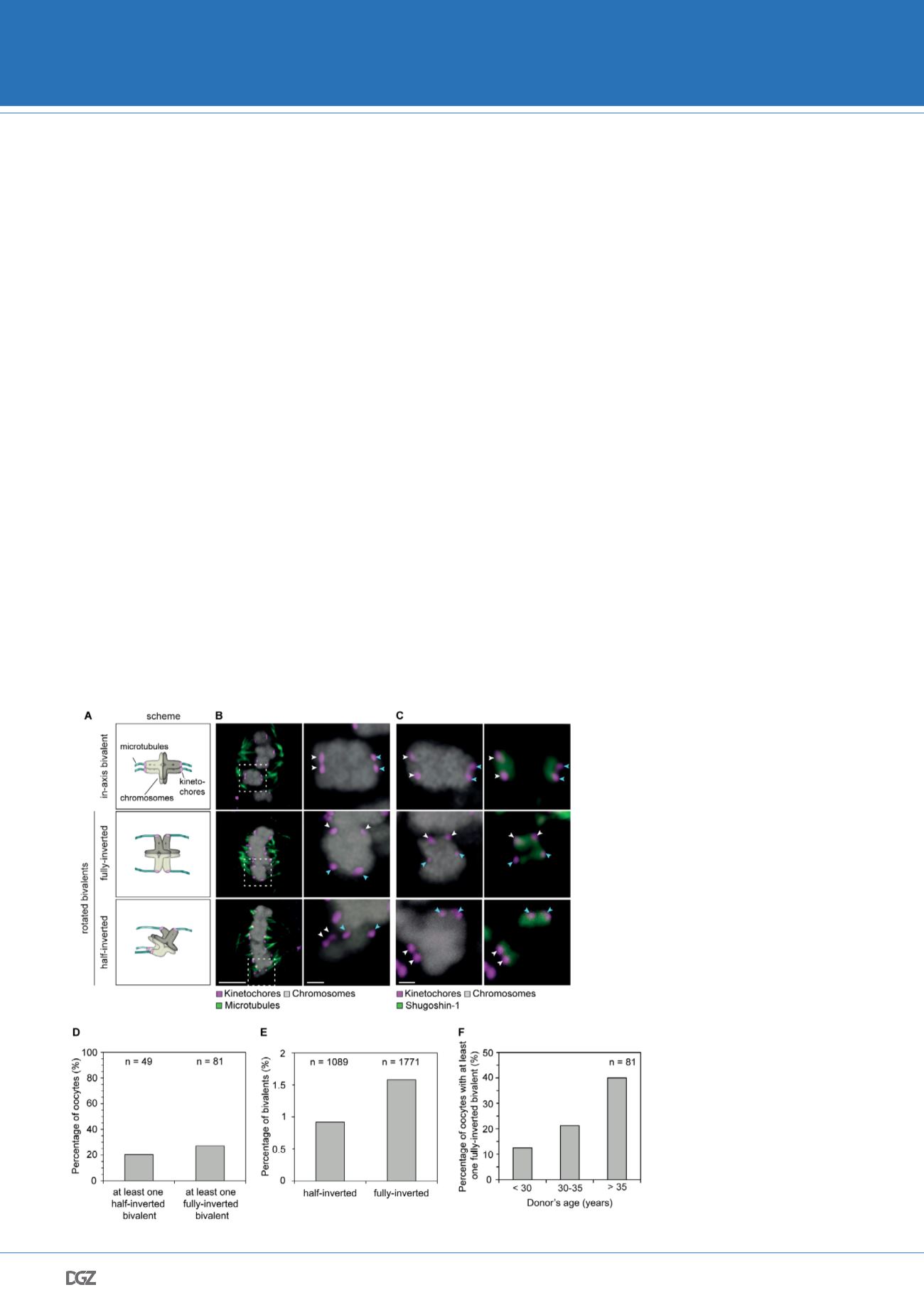
Figure 5. Sister kinetochore separa-
tion allows bivalents to rotate and
twist on the meiotic spindle.
(A) Schematic representation of the
possible orientations of bivalents on
the meiosis I spindle. (B) Representative
images of the different orientations
that bivalents can adopt relative to the
axis of the metaphase I spindle. Arrows
of the same colour highlight sister
kinetochores. Scale bar represents 5
µm, 1 µm in insets. (C) Representative
images of Shugoshin-1 staining in
bivalents rotated relative to the axis
of the metaphase I spindle. Arrows
of the same colour highlight sister
kinetochores. Scale bar represents 1
µm. (D) Occurrence of one or more
inverted bivalents in fully assembled
metaphase I spindles. Half-inverted
bivalents were scored only in oocytes
subjected to cold treatment which
selectively preserves kinetochore fibers
and hence allows for detection of
bioriented kinetochore pairs. Fully-
inverted bivalents were scored both in
cold-treated and non-treated meiosis
I spindles. (E) Proportion of bivalents
that are fully- or half-inverted on late
metaphase I spindles. (F) Occurrence of
fully inverted bivalents in oocytes from
donors across all age groups.
© Zielinska et al., Elife 2015
About the author
Melina Schuh studied biochemistry at the University of Bayreuth,
and did her Diploma thesis in the laboratory of Stefan Heidmann
and Christian F. Lehner in 2004. In 2008, she obtained her PhD in
the group of Jan Ellenberg at the European Laboratory for Mo-
lecular Biology (EMBL) and at the University of Heidelberg. She
then moved to Cambridge (UK), where she has been a group lea-
der at the MRC Laboratory for Molecular Biology from 2009 until
the end of 2015. Since January of this year, she is a Director at
the Max Planck Institute for biophysical chemistry in Göttingen,
Germany. She received several awards for her work, including the
John Kendrew Young Scientist Award, the Biochemical Society Ear-
ly Career Award (Cells) and the Lister Research Prize. She is also an
EMBO Young Investigator, an EMBO Member, and a receipient of
an ERC Starting Grant.
References
Brar, G.A., and Amon, A. (2008). Emerging roles for centromeres in meiosis I chromosome segre-
gation. Nat Rev Genet 9, 899-910.
Clift, D., and Schuh, M. (2013). Restarting life: fertilization and the transition from meiosis to
mitosis. Nat Rev Mol Cell Biol 14, 549-562.
Clift, D., and Schuh, M. (2015). A 3-step MTOC fragmentation mechanism facilitates bipolar
spindle assembly in mouse oocytes. Nat Comm 6:7217.
Courtois, A., Schuh, M., Ellenberg, J., and Hiiragi, T. (2012). The transition from meiotic to mitotic
spindle assembly is gradual during early mammalian development. J Cell Biol 198, 357-370.
Holubcová, Z., Blayney, M., Elder, K., and Schuh, M. (2015). Error-prone chromosome-mediated
spindle assembly favors chromosome segregation defects in human oocytes. Science 348,
1143-1147.
DGZ AWARD WINNERS 2016


