Cell News 04/2018
19
given adhesion structure are exposed to mechanical forces. By
combining the FL-TSM (Fig. 1c), which is characterized by an
almost digital force response, with lifetime microscopy and
advanced data analysis, we discovered that about 60–70% of
FA-resident talin molecules are mechanically loaded in FAs,
while a significant fraction did not contribute to force trans-
duction (Ringer et al. 2017).
Together, these experiments demonstrated that cells regulate
force transduction across talin in at least two complementary
ways: First, cells adjust the magnitude of force per talin mole-
cule and, second, cells modulate the amount of talin molecules
being exposed to tension. It appears that these two parameters,
molecular tension and engagement ratio, are critical determi-
nants of integrin-mediated force transduction.
Tension sensor multiplexing reveals intramolecular
tension differential across talin
A question that bothered biophysicists for some time is how
forces propagate across individual molecules in cells. For
simple proteins that mechanically engage through their N- and
C-termini and thereby act as mere connectors between two
force-bearing structures, it may be expected that mechanical
tension evenly distributes across the molecule. In the ab-
sence of experimental data on that subject, this is how force
propagation through FAs is usually modelled (Chan and Odde
2008, Elosegui-Artola et al. 2016). We wondered whether the
assumption that proteins act as ‘Hookean springs’ holds true
for complex molecules like talin that comprise multiple actin
binding sites and engage with other FA proteins (Roberts and
Critchley 2009).
A technological obstacle that prevented us from analyzing
this question in more detail was the inability to quantify the
mechanics of more than one TSM at a time. Therefore, we
developed a pair of orthogonal TSMs that can be excited by the
same wavelength but whose emission spectra can be spectrally
separated (Fig. 4a). In the first TSM, we used mTFP1 as a donor
fluorophore and the dark quencher ShadowG as an acceptor
(Murakoshi et al. 2015, Demeautis et al. 2017). In the second
module, we combined the long stokes shift (LSS) fluorophore
LSSmOrange (LmO) with mKate2 (Shcherbakova et al. 2012,
Demeautis et al. 2017). Indeed, live cell FLIM experiments
demonstrated that those multiplexing TSMs are unaffected by
each other’s presence and can be used orthogonally (Ringer et
al. 2017). Next, we inserted the first TSM into an N-terminal
region of talin (at amino acid 447), generated a second talin-1
tension sensor in which the orthogonal TSM was inserted at a
more C-terminal region (at amino acid 1973), and co-expressed
both constructs in talin-deficient cells (Fig. 4b, c). Dual color
FLIM measurements of these and control cell lines indeed re-
vealed an intramolecular tension gradient across talin (Fig. 4d):
The observed tension differential was characterized by compar-
atively high forces of more than 7 pN at the N-terminal part of
the talin-rod domain and lower tension at the C-terminal talin
region. Interestingly, the steepness of this force gradient was
detected in only a fraction of talin molecules, preferentially un-
der conditions of high myosin activity. Thus, force propagation
strongly depends on the molecule of interest and its subcellular
regulation.
Outlook
The examples above show that the molecular processes under-
lying force transduction are much more complex than previ-
ously thought. Cells adjust how many molecules are exposed
to mechanical forces, they modulate the magnitude of tension
per protein, and tune how mechanical signals are propagated
across the molecule. The developed tension sensor technique
can help unraveling these processes in a quantitative fashion,
and recent work by a growing number of colleagues shows
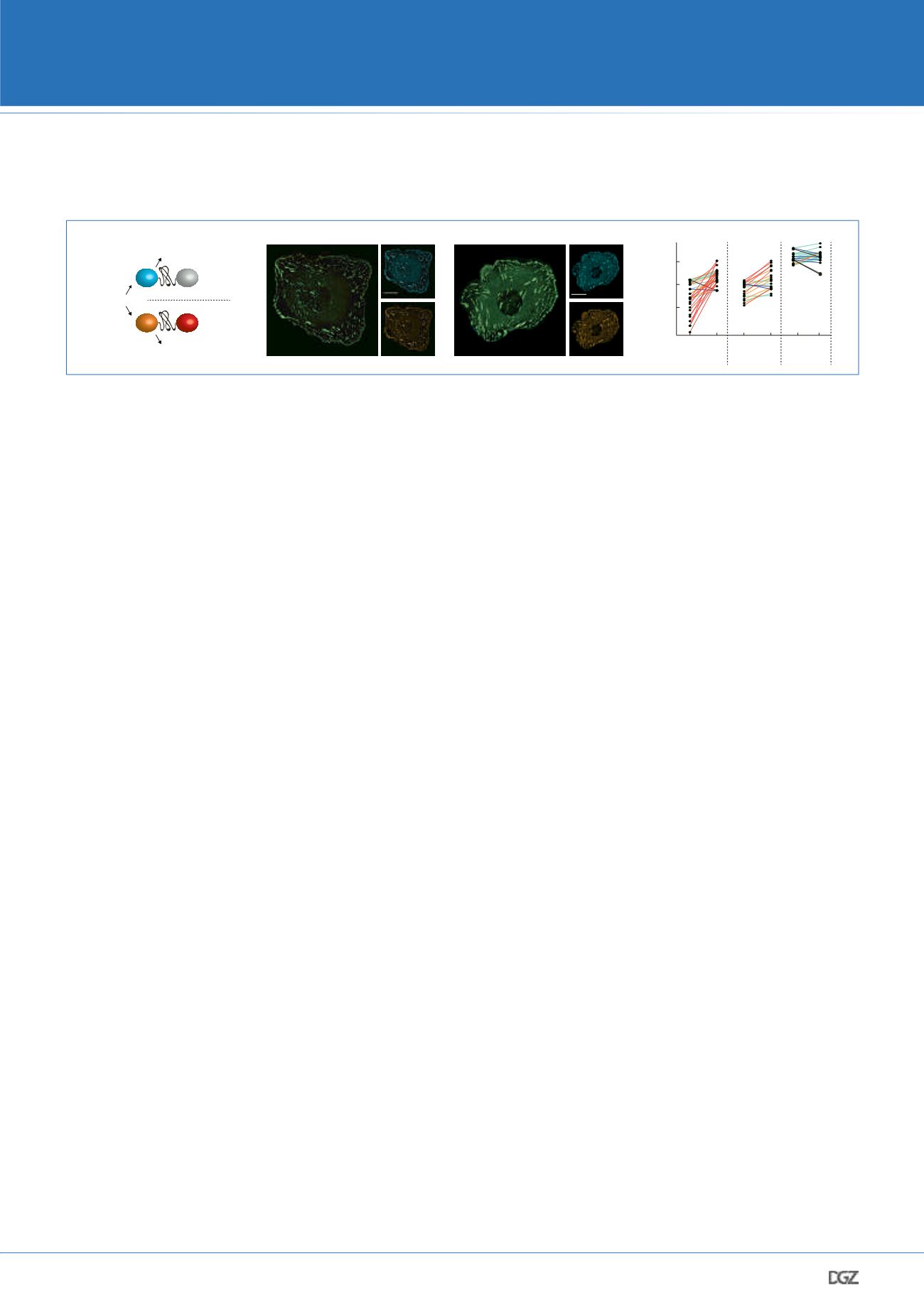
Figure 4: Tension sensor multiplexing. a. A set of orthogonal tension sensors can be used to evaluate the molecular mechanics of two proteins
simultaneously. Both constructs are excited by the same wavelength at 440 nm but their donor emission spectra can be spectrally separated. This
allows simultaneous measurements by dual color FLIM. b. To investigate intramolecular force propagation across talin, we generated two talin
tension sensor constructs. In the first sensor, TSM was inserted at the N-terminal region at aa 447; in the second construct, TSM was inserted at a
more C-terminal region (aa 1973). Both constructs were co-expressed in talin-deficient cells and localized into common subcellular structures. c.
As a control, we performed an experiment in which the positions of the TSMs were switched. d. Data from both experiments suggested that talin
is exposed to high tension at N-terminal but lower forces in C-terminal regions. Additional control experiments confirmed the specificity of the
observation.
a
b
d
c
TFP
LmO
talin TFP-FL (aa447)
LmO
TFP
talin Lmo-FL (aa1973)
FRET efficiency [%]
20
10
0
TFP ShG
LmO
mK2
ex: 440 nm
em: 490 nm
em: 560 nm
1973
LmO
447
TFP
1973
TFP
447
LmO
Con
LmO
Con
TFP
TFP-
FL
LmO-
FL
talin TFP-FL (aa1973)
talin Lmo-FL (aa447)
BINDER INNOVATION PRIZE 2018


