Cell News 04/2018
13
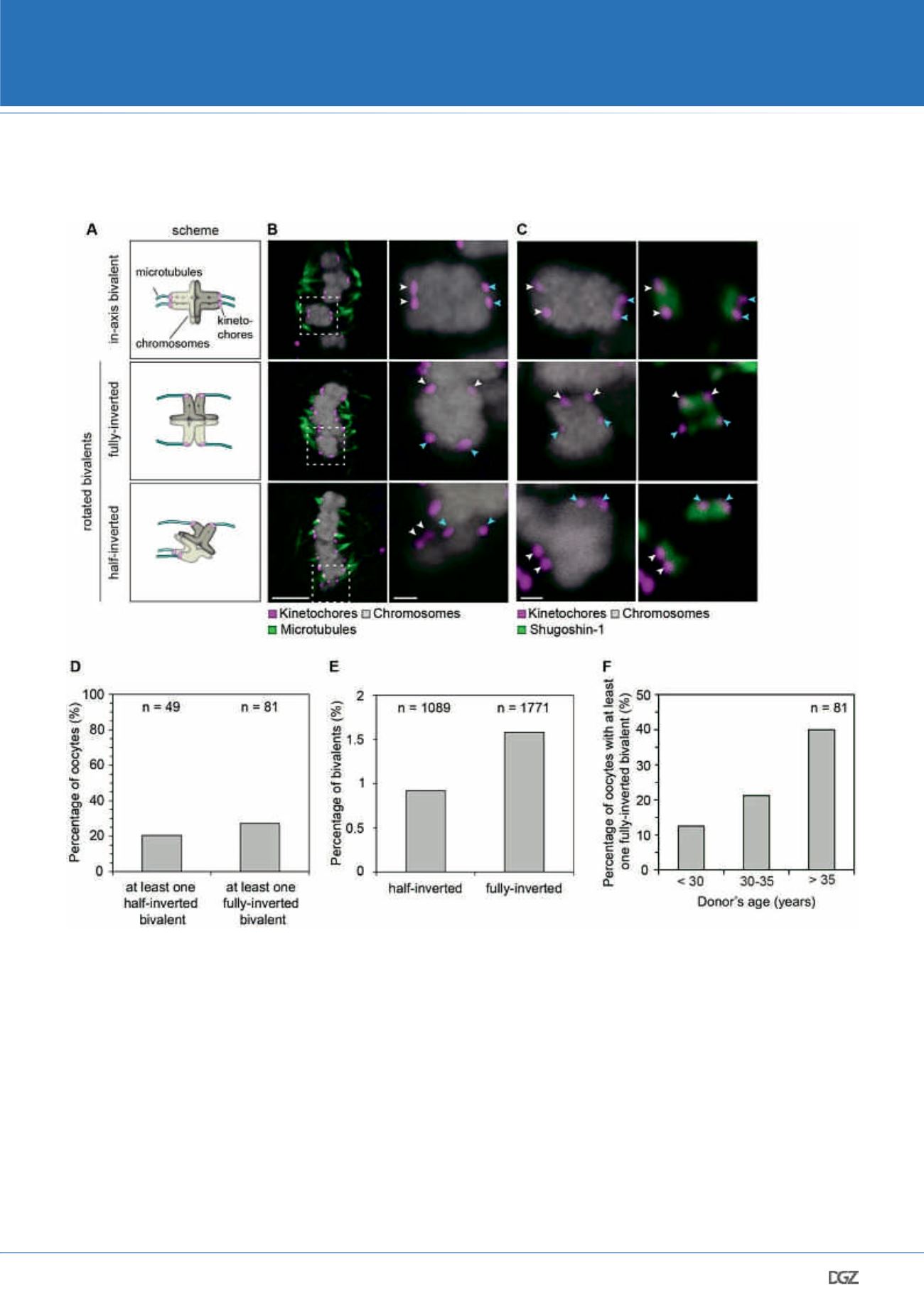
Figure 4: The pronounced sister kinetochore separation allows human bivalents to rotate on the meiosis I spindle
(A) Schematic illustration demonstrating the possible orientation of bivalents
on the spindle.
(B) Representative images of immunolabelled bivalents adopting the confor-
mation highlighted in (A). Arrows of different colours point to the two sister
kinetochore pairs. Scale bar represents 5 µm in overview and 1 µm in insets.
(C) Shugoshin-1 labelling, which demonstrates which two kinetochores within
a bivalent are sisters, in bivalents oriented in axis (top panel), fully-inverted
bivalents (middle panel) and half-inverted bivalents (bottom panel). In in-axis
bivalents, Shugoshin-1 labelling of a sister kinetochore pair is perpendicular to
the long spindle axis, whereas in fully inverted bivalents it is in parallel to the
long spindle axis. Arrows of different colours point to the two sister kineto-
chore pairs. Scale bar represents 1 µm.
(D) Frequency of half-inverted and fully-inverted bivalents in late metaphase
I human spindles. Only CSA spindles have been used to assess the presence of
half-inverted bivalents, because the selective labelling of kinetochore-bound
microtubule fibres allows for a reliable detection of a bioriented sister pairs.
For fully- inverted bivalents, both cold-treated and intact spindles have been
included in the analysis.
(E) Fraction of bivalents that exist in a half-inverted or a fully-inverted orienta-
tion during late metaphase I in humans.
(F) Occurrence of oocytes with at least one fully-inverted bivalent across the
three age groups.
NIKON YOUNG SCIENTIST AWARD 2018


