Cell News 04/2018
16
BINDER INNOVATION PRIZE 2018
Summary
The ability of cells to sense and respond to mechanical signals
is central to many biological processes (Hoffman et al. 2011,
Heisenberg and Bellaïche 2013), and a range of subcellular
structures that process mechanical information has been iden-
tified. We know, for instance, that cells probe tissue stiffness
in focal adhesions (Geiger et al. 2009), while cadherin-based
intercellular junctions transduce mechanical information
between neighboring cells (Meng and Takeichi 2009). Kineto-
chores are involved in mechanically controlling chromosome
separation during mitosis (Rago and Cheeseman 2013), and
the nucleus and its transcriptional programs are physically
connected through cytoskeletal networks with the extracellular
environment (Lombardi and Lammerding 2011). How mechan-
ical forces are transmitted across such intracellular structures
is still poorly understood. We therefore developed (Grashoff
et al. 2010) and further optimized (Austen et al. 2015, Ringer
et al. 2017) a microscopy-based technology that allows the
quantification of piconewton-scale forces in cells. The appli-
cation of this technique can provide quantitative insights into
the molecular mechanisms underlying subcellular processes of
mechanotransduction.
Introduction
It has been recognized long time ago that the vast majority
of biological processes are inherently mechanical in nature.
Walther Flemming proposed in 1880 that intracellular move-
ments are driven by mechanical forces (Flemming 1882), Julius
Wollff described in 1892 that bone tissue actively responds to
mechanical stimulation (Wolff 1892), and many developmental
biologists studied morphogenetic arrangements at the begin-
ning of the last century from a mechanical point of view (Keller
2012). Since then, conceptual frameworks have been developed
to describe a variety of biomechanical processes governing
development, tissue formation and homeostasis (Lecuit et al.
2011, Heisenberg and Bellaïche 2013). In addition, particu-
lar subcellular entities that sense and transmit mechanical
information were identified. Stretch-dependent ion channels,
for instance, are now known as evolutionary conserved sensors
of plasma membrane tension (Martinac et al. 1990), and cell
adhesion structures like focal adhesion (FAs) were found to
transduce mechanical signals during cell adhesion and mi-
gration (Pelham and Wang 1997). It was also recognized that
many organelles like the nucleus are highly mechanosensitive
and constantly integrate mechanical information in cells (Lam-
Piconewton sensitive biosensors to investigate molecular
forces in cells
Institute of Molecular Cell Biology, University of Münster, Germany
Group of Molecular Mechanotransduction, Max Planck Institute of Biochemistry, Germany
Carsten Grashoff
trap 1
trap 2
b
c
Sensitivity
15
10
5
1.0
0.5
1.5
F40
HP35
HP35st
Force [pN]
2.0
FL
high
FRET
Donor
low force
Acceptor
low FRET
Donor
high force
Acceptor
a
0
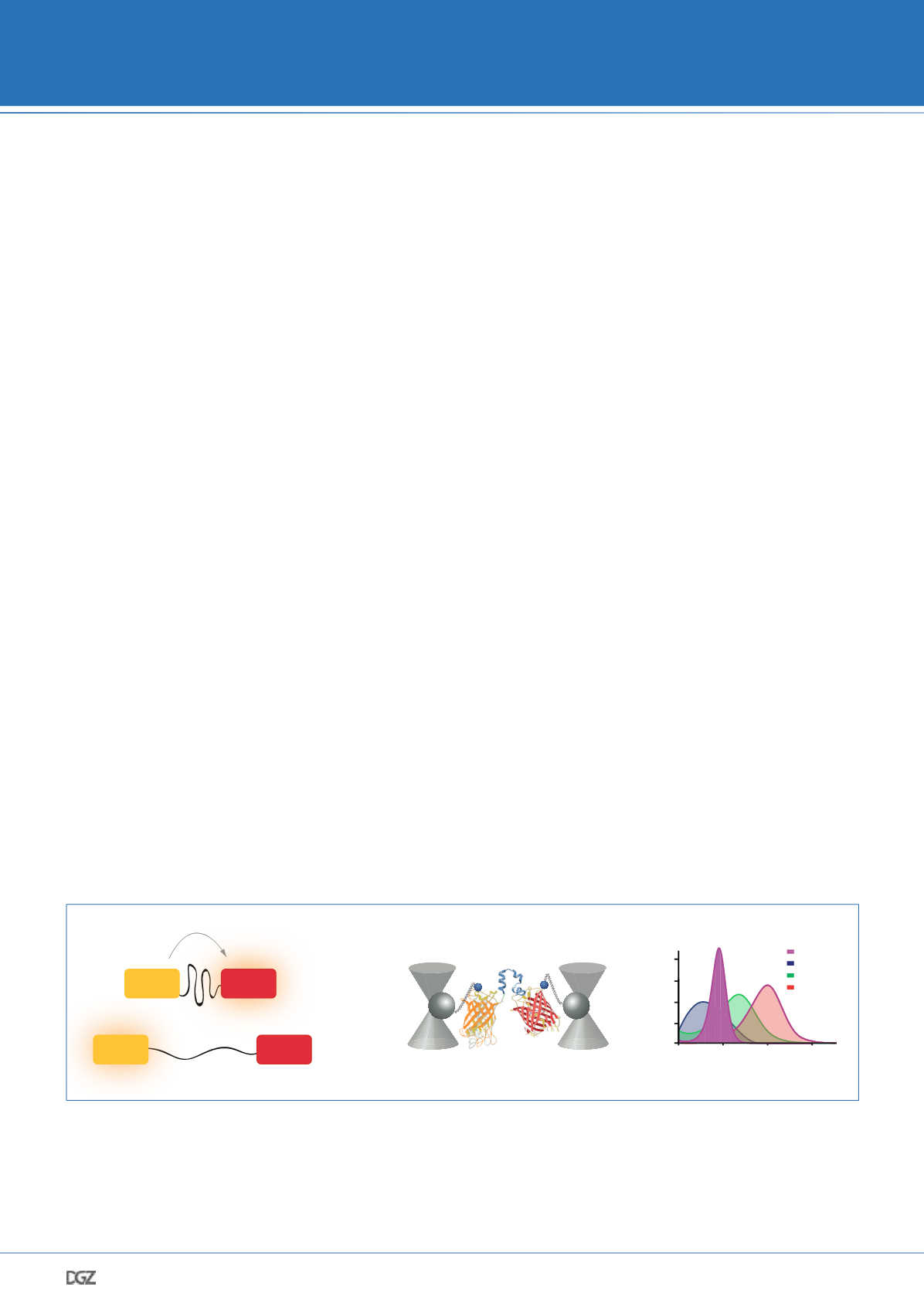
Figure 1: Single-molecule calibrated FRET-based tension sensors. a. FRET-based tension sensors comprise a donor and an acceptor fluorophore that
are connected by a mechanosensitive linker peptide. In the absence of tension, fluorophores are close to each other and undergo efficient FRET. The
linker peptide is unfolded in response to mechanical force leading to fluorophore separation and FRET loss. b. New tension sensor modules require
calibration to determine how mechanical forces affect FRET efficiency. We used single-molecule force spectroscopy to measure the force-response
characteristics and determine that sensors are reversible, loading-rate insensitive, and hysteresis-free. c. The published tension sensors modules
resolve forces ranging from 1–12 pN.


